Summary
The review summarises the evidence that Ca2+ release from sarcoplasmic reticulum (SR) is an important contributor to the systolic rise in [Ca2+]i (the Ca2+ transient) and influences the pacemaker firing rate. We believe that mechanism whereby [Ca2+]i influences firing rate is through the dependence of the Na+-Ca2+ exchanger on [Ca2+]i. Ca2+ extrusion by the electrogenic Na+-Ca2+ exchanger produces an inward current which contributes to the pacemaker currents. Confocal images of Ca2+ indicate the distribution of [Ca2+]i and Ca2+ sparks add to the evidence that the Ca2+ release from SR is involved in pacemaker activity. The normal pathway for increased heart rate is sympathetic activation; we discuss the evidence that part of the chronotropic effect of β-adrenergic stimulation is through the modulation of SR Ca2+ release. These studies show that Ca2+ handling by the pacemaker cells makes an important contribution to the regulation of pacemaker activity.
Introduction
The heart rate is determined by the firing rate of a small group of specialised pacemaker cells, which are located in the sinoatrial node in mammals and sinus venosus in amphibians. Early electrophysiological studies established that the spontaneous firing of pacemaker cells was due to a period of spontaneous diastolic depolarisation, known as pacemaker potential, which preceded the action potential. The pacemaker action potential has a relatively slow upstroke and it has long been recognised that traditional INa makes relatively little contribution (Yamagishi & Sano, 1966). Instead the L-type Ca2+ current provides the positive feedback for the rise of the action potential and the delayed rectifier potassium current is mainly responsible for repolarization. The inward currents which contribute to the slow diastolic depolarization are the key to understanding the pacemaker activity and the currents involved are still the subject of debate (Campbell et al., 1992). The hyperpolarization-activated cation current (If) has been proposed as the most important pacemaker current (DiFrancesco, 1993). However, pacemaker cells are still able to firing after blockage of If (Zhou & Lipsius, 1992) indicating that other mechanisms are involved. Several other inward currents with proposed or established roles in pacemaking include the T-type Ca2+ current (Hagiwara et al., 1988); the Na+-Ca2+ exchange current (Brown et al., 1984), background Na+ current (Hagiwara et al., 1992); persistent Na+ current (Ju et al., 1995) and the sustained inward current (Guo et al., 1995). At present there is no consensus on which of these currents makes the major contribution to pacemaking activity (compare DiFrancesco, 1993; Irisawa et al, 1993).
Given the uncertainty about which membrane current is the true pacemaker current, there is growing interest in the influence of intracellular Ca2+ on the pacemaker activity. One important issue is the possible role of Ca2+ release from the sarcoplasmic reticulum (SR) in pacemaker function. In this short review we first provide the evidence that cane toad pacemaker cells contain SR which is capable of Ca2+ release and contributes to the Ca2+ transient in pacemaker cells. We then try to establish answers to the following questions. Can spontaneous action potentials be generated in the absence of SR Ca2+ release? What is the membrane current that underlies the Ca2+-dependence of pacemaker firing rate? Is the increase in firing rate caused by β-adrenergic stimulation also mediated by the increase in Ca2+ transients that they cause?
Evidence that intracellular Ca2+ influences the firing rate of pacemaker cells.
It has long been recognised that changes in intracellular Ca2+ concentration ([Ca2+]i) affect some of the pacemaker currents and may therefore potentially affect the firing rate (DiFrancesco &Noble, 1985; Campbell et al., 1992). For instance the following potential pacemaker currents are affected by [Ca2+]i; L-type Ca current (Irisawa et al., 1993); If (Hagiwara & Irisawa, 1989); delayed rectifier potassium current (Nitta et al., 1994); sustained inward current (Guo et al., 1995). However the discovery that ryanodine, which interferes with Ca2+ release from the SR, slows the firing rate of pacemaker cells has been a major factor in the increased interest in Ca2+-dependent mechanisms (Rubenstein & Lipsius, 1989; Rigg & Terrar, 1996; Hata et al., 1996; Satoh, 1997).
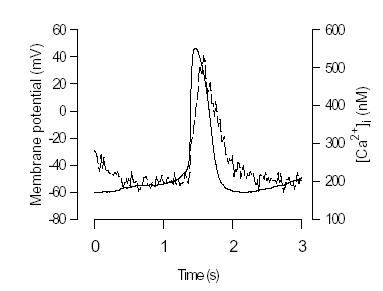
The realisation that [Ca2+]i may affect firing rate of pacemaker cells has led to new interest in measuring [Ca2+]i in pacemaker cells (Hancox et al., 1994; Li et al., 1997; Huser et al., 2000). We began to study intracellular Ca2+ in spontaneously firing toad sinus venosus (SV) pacemaker cells in 1996. There are several reasons for using toad pacemaker cells. Firstly, the sinus venosus is easy to identify in amphibian heart and provides a relatively large number of homogeneous pacemaker cells. Secondly, amphibian pacemaker cells from the toad Bufo marinus like those from the bullfrog lack If (Shibata & Giles, 1985; Ju et al., 1995) demonstrating that If is not the sole pacemaker current and providing an impetus to identify the role of other pacemaker mechanisms. Thirdly, there are quantitative amphibian models of pacemaker activity which offer the possibility of determining the relative contribution of various pacemaker currents (Rasmusson et al., 1990). Single cells were isolated and loaded with the acetoxy-methyl ester form of indo-1. Pacemaker action potential and [Ca2+]i signal were simultaneously recorded by using nystatin perforated-patch technique as shown in Figure 1. Note the rapid transient rise of [Ca2+]i (the Ca2+ transient) following the spontaneous action potential. The minimum [Ca2+]i during diastole was around 200 nM while the peak of the Ca2+ transient was around 600 nM (Ju & Allen, 1998). Although the [Ca2+]i rise was associated with action potential, the source of Ca2+ was uncertain. [Ca2+]i rise could entirely due to the influx of Ca2+ from extracellular space though voltage-sensitive Ca2+ channels in amphibian preparations (as discussed below). Therefore, it is important to demonstrate whether there are contributions from SR Ca2+ release or other possible sources, such as the reverse mode of the Na+/Ca2+ exchanger (Na+ extrusion, Ca2+ entry).

Figure 1. Simultaneously recorded action potential (solid line) and [Ca2+]i signals ( dashed line) from
single spontaneously firing toad pacemaker cell. Action potential was recorded using the nystatin perforated-patch technique. The cell was loaded with Ca2+ indicator, indo-1 AM (from Ju & Allen, 1998).
Is SR in the amphibian pacemaker cell capable of releasing Ca2+?
In pacemaker cells, morphological studies show the SR is relatively sparse (Duvert & Barets, 1979) and there is debate in the literature as to whether Ca2+-induced Ca2+ release exists in amphibian heart. Fabiato demonstrated Ca2+-induced Ca2+ release using skinned cardiac cells from a variety of species but it was notably absent from frog ventricular myocytes (Fabiato, 1982). Consistent with this finding, voltage clamp studies of frog ventricle showed that the Ca2+ involved in the activation of tension arose primarily from the extracellular space (Morad & Cleemann, 1987). Subsequently studies in frog atrial cells using ryanodine and caffeine suggested that some Ca2+ was stored and capable of release from SR (Tunstall & Chapman, 1994). Nevertheless the prevalent view remains that in amphibian heart tissue the SR is not a major source of Ca2+ during the normal contraction (Rasmusson et al., 1990).
In order to identify whether SR is capable of storing Ca2+ in cane toad pacemaker cells, we used rapid application of caffeine. Caffeine increases the frequency and duration of SR Ca2+ release channel opening (Rousseau & Meissner, 1989) and therefore rapidly depletes the SR of Ca2+ (Callewaert et al., 1989). These properties have made caffeine a popular tool to measure SR Ca2+ content in mammalian cardiac tissues (Diaz et al., 1997). In toad pacemaker cells caffeine caused a larger and rapid rise in [Ca2+]i which then fell spontaneously in the continuing presence of caffeine (Fig. 2). The peak of caffeine-induced [Ca2+]i signal was about 5 times the spontaneous [Ca2+]i transient induced by the action potential (Ju & Allen, 1999a). It is interesting that after application of caffeine, spontaneous firing stopped. The time for recovery of firing was about 20s. This time might reflect the duration of SR refilling with Ca2+ (Hussain & Orchard, 1997) and suggested that spontaneous firing was at least partly dependent on SR Ca2+ content.
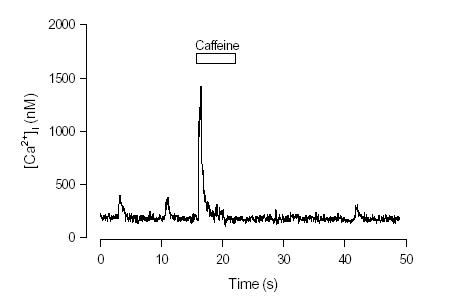
Figure 2. Effect of rapid application of caffeine on [Ca2+]i and firing rate in an isolated pacemaker
cell. Caffeine caused a large increase in [Ca2+]i which spontaneously declined in the continuing
presence of caffeine. After caffeine was washed off the cell did not fire spontaneously for about 20 s.The firing rate is indicated by the frequency of Ca2+ transients ( From Ju & Allen, 1999).
Is SR Ca2+release involved in pacemaker activity?
Although the experiments with caffeine above demonstrate that SR of toad pacemaker cells are capable of releasing Ca2+, they do not identify whether release of Ca2+ from the SR occurs during the normal action potential. To test this possibility, we used ryanodine which is an SR Ca2+ release channel blocker (Fleischer & Inui, 1989). We found that 5 min after application of 10 μM ryanodine, the peak of the Ca2+ transient decreased to 50% of control level (Fig. 3). Cells were still able to firing at this stage though at a reduced frequency. After 30 min exposure to ryanodine, spontaneous firing ceased. This effect of ryanodine on pacemaker activity is consistent with the idea that the Ca2+ transients consist of a component of Ca2+ release from SR. Decreasing SR Ca2+ release slows the heart rate. The caffeine experiments show that when the SR is emptied of Ca2+ firing temporarily ceases while the ryanodine experiments show that preventing SR Ca2+ release also slows pacemaker firing. Thus normal SR Ca2+ release seems to be needed for regular firing of the pacemaker cells. Furthermore, the argument for involvement of the SR is strengthened by recent observations of single Ca2+ release events (Ca2+ sparks) during pacemaker action potential (Huser et al., 2000; Ju & Allen, 2000a) (as described below) .
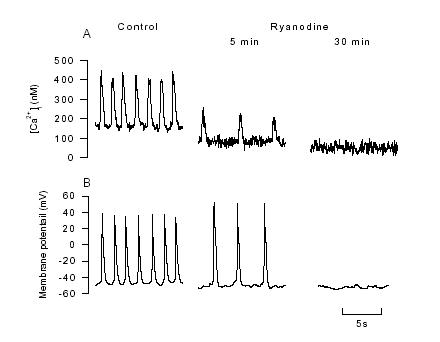
Figure 3. The effects of 10 μM ryanodine on [Ca2+]iand the spontaneous action potential. A,
[Ca2+]i transient recorded under control and after 5, and 30 min exposure to ryanodine (10 μM). B,
the effects of ryanodine on spontaneous action potential were recorded from a non-indo loaded cell to avoid the possible effect of indo-1 AM loading on pacemaker activity (from Ju & Allen, 1998).
The role of Na+-Ca2+exchanger in pacemaker activity
We have established that SR Ca2+ release occurs in pacemaker cells and that when it is prevented firing rate slows. However, the nature of the link between Ca2+ release from SR and diastolic depolarisation needs to be established. How does Ca2+ release from SR generate an inward current during the pacemaker potential? It is known that Na+-Ca2+ exchanger exist in most cardiac cells. It is also known that the Na+-Ca2+ exchanger generates a electrogenic current, since the coupling ratio for Na+-Ca2+ is 3 Na+/Ca2+ (Reeves & Hale, 1984). The amplitude and the direction of exchanger current depend most directly on the membrane potential and on [Ca2+]i . Under most normal condition the exchanger extrudes Ca2+ from the cell and therefore generates an inward current (Brown et al., 1984; Zhou & Lipsius, 1993). Although the possibility for INaCa to have a role in pacemaker activity is clear the actual importance remains controversial (Janvier & Boyett, 1996).
To establish the role of Na+-Ca2+ exchanger toad pacemaker cells we first demonstrated that
there is a very active Na+-Ca2+ exchanger by monitoring [Ca2+]i in response to Na+
free extracellular
solution (Ju & Allen, 1998). To quantify the amplitude of exchanger current that is generated by Ca2+
release from SR we simultaneously recorded [Ca2+]i and the inward current induced by a rapid
application of caffeine (Fig. 4 ). The application of caffeine produced an increase in [Ca2+]i and an
inward current. The shape and time course of the two are similar. In the presence of the Na+-Ca2+
exchanger blocker Ni2+, the caffeine-induced inward current was largely suppressed and the time
course of decay of [Ca2+]i became much slower. These results are consistent with the current and the
decline of [Ca2+]i both being caused by INaCa.
By plotting the caffeine-induced inward current versus
[Ca2+]i , we estimated that exchanger would produce about 20-27 pA inward INaCa, at the early
diastolic [Ca2+]i level (250-300 nM), 12 pA at the late diastolic [Ca2+]i level (200 nM) (Ju & Allen,
1998). Since pacemaker cells have very high input resistance, this amount of inward current would make a substantial contribution to diastolic depolarisation (DiFrancesco, 1993).
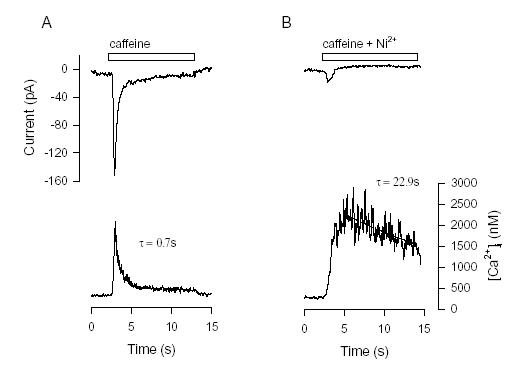
Figure 4. The inward Na+-Ca2+ exchanger current induced by Ca2+ release from SR. A, An indo-1 loaded cells was voltage- clamped at -60 mV. Rapid application of 10 mM caffeine induced an inward current (upper panel) with the time course similar to that of [Ca2+]i ( lower panel). Line drawn through declining phase of [Ca2+]i is an exponential fit whose time constant (τ) is shown. B, Caffeine and 5 mM Ni2+ applied simultaneously. The inward current was largely blocked while the [Ca2+]i increase was larger but declined more slowly. Exponential fit to early [Ca2+]i decline is shown by line and time constant (τ) (from Ju & Allen, 1998).
Given that SR Ca2+ release contributes pacemaker function at least in part through stimulating the Na+-Ca2+ exchanger, it becomes of interest to know the distribution of [Ca2+]i during the action potential. This is because the Na+-Ca2+ exchanger is situated in the surface membrane and is sensitive only to the near membrane [Ca2+]i. This issue was examined using confocal microscopy during spontaneous firing of isolated pacemaker cells. For these studies, pacemaker cells were loaded with fluo-3. Surprisingly, given that the pacemaker cells have no T-tubules we found that the distribution of Ca2+ release during an action potential was uniform (Ju & Allen, 2000a). This is surprising because one would expect the Ca2+ distribution resulting from L-type Ca channels to be localised around the edges of the cell. In fact Ca2+ reached a similar peak in the centre of the cell as at the edge and there was no detectable delay in the rise of Ca in the middle of the cell compared to the edge. One explanation for these findings is that SR is uniformly distributed across the cell and the triggering mechanism is so fast that no detectable decay occurs between edge and centre of these small 4 μm diameter cells.
Confocal studies of [Ca2+]i are also capable of localised, spontaneous Ca2+ release from SR release channels (Ca2+ sparks) which provide further information about Ca2+ release from the SR. Ca2+ sparks were detected in cane toad cells and become smaller in magnitude and longer in duration in the presence of 250 nM of ryanodine (Ju & Allen, 2000a). This finding is consistent with the ability of low concentration of ryanodine to cause the SR Ca2+ channels to enter an intermediate conductance state with long openings (Rousseau et al., 1987). A novel finding was that the frequency of Ca2+ sparks increased just before an action potential. A recent study in mammalian pacemaker cells has confirmed this finding and suggested that the mechanism involved is that T-type Ca2+ current triggers Ca2+ sparks from SR close to the membrane (Huser et al., 2000). We do not believe this is the only mechanism involved because in our experiments the increased frequency of sparks was also observed in the middle of the cell.
What is a trigger for SR Ca2+release in pacemaker cells?
In order to study the mechanism underlying SR Ca2+ release in pacemaker cells, we simultaneously voltage-clamped the cells and measured [Ca2+]i. In the present of SR Ca2+ pump inhibitor 2,5-di(tert-butyl)-1,4-hydroquinone (TBQ), which would be expected to deplete the SR of Ca2+. Ca2+ transients were reduced to 34% while there was no significant effect on the peak inward current. This result suggests that about 66% of Ca2+ contributing to the Ca2+ transient is released from SR, which is consistent with previous observation in spontaneous firing cells with ryanodine. In response to a series of membrane depolarisations we found that the amplitude of the Ca2+ transient is not simply related to the size of inward current (Ju & Allen, 2000b). Ca2+ transients increased continuously as membrane potential was increased whereas the current-voltage relationship of the inward current was bell-shaped. By using various channel blockers we found that not only L-type Ca2+ current but also reversal mode Na+-Ca2+ exchanger current could trigger Ca2+ SR release in pacemaker cells (Ju & Allen, 2000b). The results pose the question whether reversal model Na+-Ca2+ exchanger induces Ca2+ induced Ca2+ release during the spontaneous pacemaker action potentials. However, lack a specific Na+-Ca2+ exchanger blocker prevents us addressing this issue directly at present.
Is the increase heart rate by adrenaline related to the change of SR Ca2+release?
It is generally thought that the increase in the heart rate after β-adrenergic stimulation is caused by modulation of ionic current, such as L-type Ca2+ current (Noma et al., 1980) and If (DiFrancesco, 1981). It is also known that β-adrenergic stimulation increase the amplitude of Ca2+ transients in cardiac myocytes (Allen & Blinks, 1978; Hussain & Orchard, 1997; Hancox et al., 1994). We have found that in toad pacemaker cells various aspects of Ca2+ handling were modified by β-adrenergic stimulation, including increases in the L-type Ca2+ current, the SR Ca2+ content, and the magnitude of Na+-Ca2+ exchanger current (Ju & Allen, 1999a). We also found that increased Na+-Ca2+ exchange current could be explained by the increased [Ca2+]i rather than changes in the intrinsic properties of exchanger (Ju & Allen, 1999b). Since adrenaline changed several potential pacemaker currents in addition to having multiple effects on the [Ca2+]i handling, it is difficult to identify the exact basis of the chronotropic effect. However, one intriguing observation suggests that SR Ca2+ release has a critical role in β-adrenergic stimulation. We found that isoprenaline was able to restore spontaneous firing in the cells treated with a high concentration of ryanodine but not in the cells treated with a low concentration of ryanodine (Ju & Allen, 1999a). It is known that different concentrations of ryanodine have different effect on the SR Ca2+ release channel (Fleischer & Inui, 1989). Low concentration of ryanodine lead to channels open in the subconductance state whereas high concentration of ryanodine close the channels. Thus, we expect the SR to be empty of Ca2+ at low ryanodine concentrations but loaded with Ca2+ at high ryanodine concentration and this prediction was confirmed by caffeine exposures. It appears that isoprenaline was able to overcome the inhibition of Ca2+ release caused by high ryanodine concentration and that spontaneous firing could resume provide SR Ca2+ release could occur. In contrast, when intracellular Ca2+ store were emptied by low concentration of ryanodine, spontaneous firing was unable to occur.
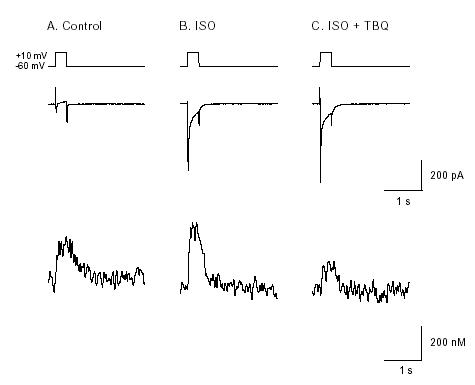
Figure 5. Membrane current and [Ca2+]i in a voltage-clamped pacemaker cells showing effects of
isoprenaline and TBQ. Cell was loaded with indo-1 AM. Perforated patch technique was used to voltage clamp cells. Depolarisation to 0 mV from holding potential -60 mV evoked an inward current associated with an [Ca2+]i transient in the control condition (A). B, 2
μ M isoprenaline caused a larger
increase of inward current and [Ca2+]i transient. C, after 5min application of 10
&muM TBQ in the
continuing presence of isoprenaline. The amplitude of [Ca2+]i transient was greatly decreased while
the amplitude of inward current remained the same (from Ju & Allen, 1999).
The above experiments suggest that SR Ca2+ release plays a specific role in response to β-stimulation. In order to separate the effects of β-stimulation on Ca2+ influx from that due to SR Ca2+ release we simultaneously recorded Ca2+ current and [Ca2+]i . We found that in the present of isoprenaline both Ca2+ current and [Ca2+]i transients were increased (Fig. 5). TBQ was used to reveal the SR contribution. We found that application of TBQ had no significant effect on Ca2+ current enhanced by isoprenaline. However, [Ca2+]i transient was greatly decreased. The similar result was obtained by using low concentrations of ryanodine. Such experiments suggest that SR Ca2+ release contributes about 50% of the Ca2+ transient both in the absence and presence of β-adrenergic stimulation (Ju & Allen, 1999a). Therefore the increase of [Ca2+] transient by β-stimulation is partly caused by increased SR Ca2+ release. In order to maintain the homeostasis of [Ca2+]i , the Na+-Ca2+ exchanger would produce more inward current by extruding more Ca2+. Thus increased inward current during the diastolic potential would accelerated the diastolic depolarisation, therefore increasing the heart rate.
Conclusion
The evidence is clear that [Ca2+]i and SR Ca2+ release are in some way related to the firing rate of cane toad pacemaker cells. It is very likely that INaCa is at least part of the intermediary process which links the Ca2+ to the pacemaker current. However many other details are less clear; does [Ca2+]i affect other pacemaker currents which have significant effects? Are Ca2+ sparks important in the pacemaker process and is the mechanism proposed by Huser et al. (2000) correct and applicable in other cell types? Does SR Ca2+ release have some special role over and above its contribution to the Ca2+ transients? The ryanodine experiments suggest that it may and studies by Cousins & Bramich (1998) also suggest there may be a class of Ca2+ store which is modulated only by neuronally-released adrenaline.
Cellular studies of pacemaker cells have been impeded by the small numbers of these cells and the difficulties in isolating them. There is increasing evidence that pacemaker function declines in the elderly and those with ischaemic heart disease (Benditt et al., 1995) and understanding and treatment of these problems is dependent on increasing understanding of pacemaker function at a cellular and molecular level. Acknowledgements.
We are grateful for support from the National Health and Medical Research Council of Australia.
References
Allen, D.G. & Blinks, J.R. (1978) Calcium transients in aequorin-injected frog cardiac muscle. Nature, 273, 509-513.
Benditt, D.G., Sakaguchi, S., Golstein, M.A., Lurie, K.G., Gornick, C.C. & Adler, S.W. (1995) Sinus Node Dysfunction: Pathophysiology, clinical feature, evaluation, and treatment. In: Cardiac electrophysiology: from cell to bedside.
Brown, H.F., Kimura, J., Noble, D., Noble, S.J. & Taupignon, A. (1984) The slow inward current, isi, in the rabbit sino-atrial node investigated by voltage clamp and computer simulation. Proceedings of the Royal Society of London. Series B: Biological Sciences, 222, 305-328.
Callewaert, G., Cleemann, L. & Morad, M. (1989) Caffeine-induced Ca2+ release activates Ca2+ extrusion via Na+-Ca2+ exchanger in cardiac myocytes. American Journal of Physiology, 257, C147-52.
Campbell, D.L., Rasmusson, R.L. & Strauss, H.C. (1992) Ionic current mechanisms generating vertebrate primary cardiac pacemaker activity at the single cell level: an integrative view. Annual Review of Physiology, 54, 279-302.
Cousins, H.M. & Bramich, N.J. (1998) Effects of sympathetic nerve stimulation on membrane potential, [Ca2+]i and force in the arrested sinus venosus of the toad, Bufo marinus. Journal of Physiology, 505, 513-527.
Diaz, M.E., Trafford, A.W., O'Neill, S.C. & Eisner, D.A. (1997) Measurement of sarcoplasmic reticulum Ca2+ content and sarcolemmal Ca2+ fluxes in isolated rat ventricular myocytes during spontaneous Ca2+release. Journal of Physiology, 501 ( Pt 1), 3-16.
DiFrancesco, D. (1981) A study of the ionic nature of the pace-maker current in calf Purkinje fibres. Journal of
Physiology, 314, 377-393.
DiFrancesco, D. (1993) Pacemaker mechanisms in cardiac tissue. Annual Review of Physiology, 55, 455-472.
DiFrancesco, D. & Noble, D. (1985) A model of cardiac electrical activity incorporating ionic pumps and concentration changes. Philosophical Transactions of the Royal Society of London - Series B: Biological Sciences, 307, 353-398.
Duvert, M. & Barets, A.L. (1979) Fine structure and organization of the sarcoplasmic reticulum in the sino-
atrial fibres of the frog heart. Zeitschrift fur Naturfurschung, 34, 865-875.
Fabiato, A. (1982) Calcium release in skinned cardiac cells: variations with species, tissues, and development. Federation Proceedings, 41, 2238-2244.
Fleischer, S. & Inui, M. (1989) Biochemistry and biophysics of excitation-contraction coupling. Annual Review of Biophysics & Biophysical Chemistry, 18, 333-364.
Guo, J., Ono, K. & Noma, A. (1995) A sustained inward current activated at the diastolic potential range in rabbit sino-atrial node cells. Journal of Physiology, 483, 1-13.
Hagiwara, N. & Irisawa, H. (1989) Modulation by intracellular Ca2+ of the hyperpolarization- activated inward current in rabbit single sino-atrial node cells. Journal of Physiology, 409, 121-141.
Hagiwara, N., Irisawa, H. & Kameyama, M. (1988) Contribution of two type of calcium currents to the pacemaker potentials of rabbit sinoatrial node cells. Journal of Physiology, 395, 233-253.
Hagiwara, N., Irisawa, H., Kasanuki, H. & Hosoda, S. (1992) Background current in sino-atrial node cells of the rabbit heart. Journal of Physiology, 448, 53-72.
Hancox, J.C., Levi, A.J. & Brooksby, P. (1994) Intracellular calcium transients recorded with Fura-2 in spontaneously active myocytes isolated from the atrioventricular node of the rabbit heart. Proceedings of the Royal Society of London - Series B: Biological Sciences, 255, 99-105.
Hata, T., Noda, T., Nishimura, M. & Watanabe, Y. (1996) The role of Ca2+ release from sarcoplasmic reticulum in the regulation of sinoatrial node automaticity. Heart and Vessels, 11, 234-241.
Huser, J., Blatter, L. A. & Lipsius, S.L. (2000) Intracellular Ca2+ release contributes to automaticity in cat atrial pacemaker cells [see comments]. Journal of Physiology, 524 Pt 2, 415-422.
Hussain, M. & Orchard, C.H. (1997) Sarcoplasmic reticulum Ca2+ content, L-type Ca2+ current and the Ca2+ transient in rat myocytes during β-adrenergic stimulation. Journal of Physiology, 505, 385-402.
Irisawa, H., Brown, H.F. & Giles, W. (1993) Cardiac pacemaking in the sinoatrial node. Physiological Reviews, 73, 197-227.
Janvier, N.C. & Boyett, M.R. (1996) The role of Na-Ca exchange current in the cardiac action potential. Cardiovascular Research, 32, 69-84.
Ju, Y.K. & Allen, D.G. (1998) Intracellular calcium and Na+-Ca2+ exchange current in isolated toad pacemaker cells. Journal of Physiology, 508, 153-166.
Ju, Y. K. & Allen, D.G. (1999b) Does adrenaline modulate the Na+/Ca2+ exchanger in isolated toad pacemaker cells. Pflügers Archiv European Journal of Physiology, 438, 338-343.
Ju, Y.K. & Allen, D.G. (1999a) How does β-adrenergic stimulation increase heart rate? The role of intracellular Ca2+ release in amphibian pacemaker cells. Journal of Physiology, 516, 793-804.
Ju, Y.K. & Allen, D.G. (2000a) The distribution of calcium in toad cardiac pacemaker cells during spontaneous firing. Pflügers Archiv European Journal of Physiology, 441, 219-227.
Ju, Y.K. & Allen, D.G. (2000b) The mechanisms of sarcoplasmic reticulum Ca2+ release in toad pacemaker cells. Journal of Physiology, 525 Pt 3, 695-705.
Ju, Y.K., Saint, D.A., Hirst, G.D. & Gage, P.W. (1995) Sodium currents in toad cardiac pacemaker cells. Journal of Membrane Biology, 145, 119-128.
Li, J., Qu, J. & Nathan, R.D. (1997) Ionic basis of ryanodine's negative chronotropic effect on pacemaker cells isolated from the sinoatrial node. American Journal of Physiology, 273, H2481-H2489.
Morad, M. & Cleemann, L. (1987) Role of Ca2+ channel in development of tension in heart muscle. Journal of
Molecular and Cellular Cardiology, 19, 527-553.
Nitta, J., Furukawa, T., Marumo, F., Sawanobori, T. & Hiraoka, M. (1994) Subcellular mechanism for Ca(2+)-
dependent enhancement of delayed rectifier K+ current in isolated membrane patches of guinea pig ventricular myocytes. Circulation Research, 74, 96-104.
Noma, A., Kotake, H. & Irisawa, H. (1980) Slow inward current and its role mediating the chronotropic effect of epinephrine in the rabbit sinoatrial node. Pflügers Archiv European Journal of Physiology, 388, 1-9.
Rasmusson, R.L., Clark, J.W., Giles, W.R., Shibata, E.F. & Campbell, D.L. (1990) A mathematical model of a bullfrog cardiac pacemaker cell. American Journal of Physiology, 259, H352-H369.
Reeves, J.P. & Hale, C.C. (1984) The stoichiometry of the cardiac sodium-calcium exchange system. Journal of
Biological Chemistry, 259, 7733-7739.
Rigg, L. & Terrar, D.A. (1996) Possible role of calcium release from the sarcoplasmic reticulum in pacemaking in guinea-pig sino-atrial node. Experimental Physiology, 81, 877-880.
Rousseau, E. & Meissner, G. (1989) Single cardiac sarcoplasmic reticulum Ca2+-release channel: activation by caffeine. American Journal of Physiology, 256, H328-H333.
Rousseau, E., Smith, J.S. & Meissner, G. (1987) Ryanodine modifies conductance and gating behavior of single Ca2+ release channel. American Journal of Physiology, 253, C364-C368.
Rubenstein, D.S. & Lipsius, S.L. (1989) Mechanisms of automaticity in subsidiary pacemakers from cat right atrium. Circulation Research, 64, 648-657.
Satoh, H. (1997) Electrophysiological actions of ryanodine on single rabbit sinoatrial nodal cells. General
Pharmacology, 28, 31-38.
Shibata, E.F. & Giles, W.R. (1985) Ionic currents that generate the spontaneous diastolic depolarization in
individual cardiac pacemaker cells. Proceedings of the National Academy of Sciences USA, 82, 7796-
7800.
Tunstall, J. & Chapman, R.A. (1994) The effect of ryanodine on the contraction of isolated frog atrial trabeculae is triggered by caffeine. Experimental Physiology, 79, 435-444.
Yamagishi, S. & Sano, T. (1966) Effect of tetrodotoxin on the pacemaker action potential of sinus node. Proceedings of the Japan Academy, 42, 1194-1196.
Zhou, Z. & Lipsius, S.L. (1992) Properties of the pacemaker current (If) in latent pacemaker cells isolated from cat right atrium. Journal of Physiology, 453, 503-523.
Zhou, Z. & Lipsius, S.L. (1993) Na+-Ca2+ exchange current in latent pacemaker cells isolated from cat right
atrium. Journal of Physiology, 466, 263-285.