Giuseppe S. Posterino
Department of Zoology, Faculty of Science and Technology, La Trobe University, 3086, Victoria,
Australia.
Summary
In skeletal muscle, excitation-contraction (E-C) coupling describes a cascade of cellular events initiated by an action potential (AP) at the surface membrane which ultimately results in muscle contraction. Being able to specifically manipulate the many processes that constitute E-C coupling as well as the many factors that modulate these processes has proved challenging. One of the simplest methods of gaining access to the intracellular environment of the muscle fibre is to physically remove (mechanically skin) the surface membrane. In doing so the myoplasmic environment is opened to external manipulation. Surprisingly, even though the surface membrane is absent, it is still possible to activate both twitch and tetanic force responses in a mechanically-skinned muscle fibre by generating an AP in the transverse tubular system. This proves that all the key steps in E-C coupling are retained in this preparation. By using this technique, it is now possible to easily manipulate the myoplasmic environment and observe how altering individual factors affects the normal E-C coupling sequence. The effect of important factors, such as the redox state of the cell, parvalbumin, and the sarcoplasmic reticulum Ca2+-ATPase, on twitch and tetanic force can now be specifically investigated independent of other factors.
1. Overview of excitation-contraction coupling in skeletal muscle
Given the extensive literature on E-C coupling in skeletal muscle, a detailed examination of each step in the process is beyond the scope of this article. Instead, recent reviews are cited where appropriate and only a brief account of E-C coupling is given here to help the reader appreciate certain points raised later in this review. In this article, I will give examples of the recent use and possible future contributions of mechanically-skinned fibre technique towards the understanding certain parts of the E-C coupling cascade, namely: a) the spread of excitation within the transverse tubular (t-) system; b) the mechanisms of communication between the voltage-sensors in the t-system and the Ca2+ release channels of the terminal cisternae of the sarcoplasmic reticulum (SR); and c) Ca2+ handling by the SR.
In skeletal muscle, the AP at the surface membrane rapidly spreads down into the t-system of the muscle fibre where the associated depolarization is sensed by the voltage-sensors (dihydropyridine receptors - DHPRs) (Schneider, 1994; Melzer et al., 1995). The DHPRs of skeletal muscle are modified L-type Ca2+ channels in which the Ca2+ channel function is virtually redundant because entry of Ca2+ into the cell is not necessary to initiate contraction (Rios & Pizzaro, 1991; Dulhunty, 1992; Melzer et al., 1995). The DHPR consists of five subunits, with the α1 subunit playing the primary role in E-C coupling. The α1 of the DHPR is composed of four repeats (I-IV), each with six hydrophobic intramembranous segments (s1-s6). The fourth segment (s4) of each repeat contains a series of positive charges which are thought to be the voltage-sensitive elements that underlie the voltage-dependent asymmetric charge movement observed originally by Schneider & Chandler (1973). Connecting each repeat are hydrophilic peptide loops, with the myoplasmic loop joining repeats II and III being essential for signal transmission to the SR in vertebrate skeletal muscle (Tanabe et al., 1990). The DHPRs co-localize in arrangements of four, termed tetrads (Block et al., 1988), and are located immediately adjacent to alternate Ca2+-release channels (ryanodine receptors - RYRs) in the adjacent SR. Activation of the DHPRs subsequently leads to the activation of the RYRs by a mechanism that is not fully understood. The RYRs are large homotetrameric Ca2+ channels which tightly bind the plant alkaloid ryanodine. The RYRs specific to skeletal muscle are termed RYR1 and are arranged in closely packed arrays in vivo (Block et al., 1988). In mammalian muscle, every RYR1 appears to be functionally identical at the biochemical level (Ogawa, 1994), although the properties of DHPR coupled versus DHPR uncoupled RYR1s in vivo may differ. In amphibian muscle, the RYR1s are composed of two isoforms, α and β, of which the properties of the β-isoform may or may not be differ from amphibian α and mammalian RYR1 (Ogawa, 1994; Franzini-Armstrong & Protasi, 1997; Ogawa et al., 1999). A distinct feature of the various RYR1s of both amphibian and mammalian skeletal muscle is the strong inhibition of channel activity by physiological levels of Mg2+ (~1 mM), millimolar concentrations of Ca2+, and the ability of ATP to stimulate channel activity even in the absence of Ca2+ (Lamb, 2000). These features are essential for the type of E-C coupling observed in skeletal muscle as opposed to cardiac and smooth muscle cells.
Precisely how the DHPR and the RYR1 interact has not been established, although a direct interaction between these two channels is thought to occur (Melzer et al., 1995; Meissner & Lu, 1995; Franzini-Armstrong & Protasi, 1997). Activation of the RYR allows Ca2+ stored in the SR to enter the myoplasm where it binds to the contractile apparatus to initiate force production (Melzer et al., 1995). The release of Ca2+ is tightly controlled by the DHPRs (Rios & Pizzaro, 1991; Melzer et al., 1995). The cessation of Ca2+ release upon deactivation of the DHPRs leads to relaxation of force as the Ca2+ initially released is resequestered back into the SR through the activity of the SR Ca2+-ATPases and in fast-twitch fibres, relaxation may be aided by the binding of Ca2+ to parvalbumin (Rall, 1996).
2. Techniques for investigating E-C coupling in skeletal muscle
Many techniques have been used in the study of E-C coupling and all have both advantages and disadvantages. Whole intact cell preparations have the advantage that they retain normal physiological function. However, the usefulness of these preparations is to some extent limited by difficulties in controlling and measuring intracellular processes. One way around this problem is to use molecular biology techniques, such as the knockout of a specific gene. In this way the influence of a specific protein can be removed and the effect of this studied in the intact system. However, given the complex interaction between many cellular constituents, the removal of a specific component may cause some unintentional change in the function of other components. In contrast, the biochemical approach involves the study of key components in well controlled artificial environments, such as the isolation of a single channel in an artificial lipid bilayer. In this way the basic function of a particular component can be determined in isolation, although the effect of complex interactions with other cellular components that may normally occur in vivo is lost. Bridging the gap between intact fibre and biochemical techniques are the skinned muscle fibre preparations. The key advantage of these preparations is that the myoplasmic environment can be easily manipulated whilst in certain skinned fibre preparations (see below), all the essential elements in the E-C coupling cascade also remains intact.
3. Types of skinned fibre preparations
There are a two main ways to permeablise a skeletal muscle fibre – chemically and mechanically. However, these differ in the consequences they have on the various structures of the muscle fibre.

a) Chemical skinning
This involves the use of a number of chemical reagents that permeablise the various membranes of the fibre – some are more selective than others. Commonly used reagents are saponin, β-escin, glycerol and triton-X 100. The more selective permeablising agents (e.g. saponin and β-escin) are thought to act primarily on the surface membranes (sarcolemma) and t-tubules by binding cholesterol which is largely absent from the SR. However, this selectivity is not as precise as first thought and significant effects on the SR have been observed (Launikonis & Stephenson, 1997, 1999). Other non-specific reagents (such as triton-X 100 and glycerol) destroy all the membrane structures and leave only the contractile machinery intact. The type of permeablising reagent that is most appropriate depends on the particular cellular process the investigator wishes to examine. For examination of the properties of the contractile apparatus, it is often best to remove all membrane structures to ensure that they do not interfere with the measurements. For example, the presence of a functional SR can greatly affect the Ca2+ gradients within the fibre and this may significantly affect the force-[Ca2+] relationship unless the [Ca2+] was buffered very strongly. If on the other hand the purpose is to investigate the Ca2+-handling properties of the SR, one needs to use the more selective reagents that leave the SR intact and functional - and this may not be possible with even the more selective reagents used (cf. Launikonis & Stephenson, 1997, 1999). Thus, to avoid any undesired effects of permeablising reagents it is perhaps best to use mechanically-skinned fibres.
b) Mechanical-skinning
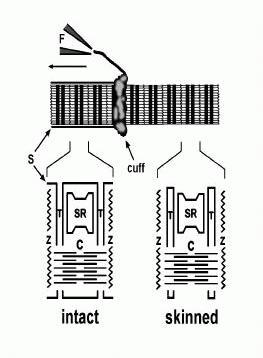
The mechanically-skinned fibre technique (originally termed Natori-type fibres) was first developed by Natori in 1954. He showed that it was possible to gain access to intracellular environment by physically rolling back the surface membrane of a single muscle fibre with a pair offine needles under paraffin oil (see Fig. 1.). Unlike chemically-skinned fibres, in which the surface membrane, t-system and SR are perforated by various chemical agents, in mechanically-skinned fibres the t-system seals off to form an intact, fully functional compartment (see later). The SR also remains intact and fully functional. There are other variations of this technique, such as splitting the fibre, however, the t-system in this preparation does not seal off which limits its experimental application. The functional integrity of mechanically-skinned fibres is one of the key advantages of this preparation and this can be seen by its use in the examination of many aspects of E-C coupling.
Examination of the contractile apparatus: One of the earliest uses of mechanically-skinned fibres was in the study of the various properties of the contractile apparatus in which a number of models derived from earlier biochemical studies could be tested in a more physiological preparation (Gordon et al.,2000). These fibres were often also treated with membrane permeablising reagents, such as triton X-100, and are thus better termed chemically-skinned fibres. Nevertheless, the specific role of Ca2+,
Mg2+, ATP and other compounds in the regulating contraction has been studied extensively by
exposing the myofilaments of both mechanically-skinned and chemically-skinned fibres to various buffered solutions (Stephenson, 1981; Gordon et al., 2000). Mechanically-skinned fibres are useful in understanding the properties of the contractile apparatus as it has been shown that the intrinsic contractile properties of intact fibres can be observed in mechanically-skinned fibres provided that the buffering of various factors in solution is firmly controlled (Moisescu, 1976). More recently, a study comparing the Ca2+-activation properties (i.e. the Ca2+-sensitivity and Hill coefficient) of both intact
and mechanically-skinned fibres further demonstrate that contractile function remains unchanged following skinning (Konishi & Watanabe, 1998).
Examination of SR properties: Biochemical assays have provided enormous insight into the function of the SR and its key molecules. However, during preparation of these assays, the structure of the SR and associated proteins may be compromised and no longer representative of their state in vivo. Furthermore, the normal constraints and relationships between RYR1s and other key molecules present in vivo are typically lost (Favero, 1999). The importance of such constraints and relationships between molecules for their normal function is becoming clearer. As mentioned earlier, RYR1s are arranged in a closely packed array in vivo and it has been shown recently that RYRs will spontaneously form these arrangements in solution (Yin & Lai, 2000). Furthermore, RYRs in bilayers have been observed to open and close in synchrony (termed coupled gating, Marx et al., 1998). This phenomena suggested that there is physical cooperativity between such channels and this could well be important for normal Ca2+ release. There is also a close association between RYR1 and DHP receptors of the t-system (Block et al., 1988) and recent studies have suggested that RYR1 can bind DHP receptors in vitro (Murray & Ohlendiek, 1997). The close proximity of the DHP receptors and RYR1 appears to directly influence their individual functions (Nakai et al., 1996). Associated proteins of the RYR1, such as FKBP-12, also help to link and control neighbouring channels (Marx et al.,1998) whilst other associated molecules, such as calmodulin and calsequestrin, appear to regulate channel activity at an individual level (Franzini-Armstrong & Protasi, 1997). Mechanically-skinned fibres retain these relationships and structural constraints and are thus ideal for the examination of the activity of the RYR1 and the Ca2+-ATPase in their native state. The endogenous Ca2+ content of the SR, which is reported to regulate the activity of the RYR1 (Sitsapesan & Williams, 1997), can also be assayed and controlled in this preparation. By using a number of RYR agonists and antagonists, such as caffeine and ryanodine, as well as antagonists of the Ca2+-ATPase, such as 2,5-di(tert-butyl)-1,4-benzohydroquinone (TBQ), the properties of the SR and related molecules can be examined (see later).
Examination of voltage-dependent Ca2+release: Considering the apparent importance of maintaining normal structural integrity, how do we really know that mechanically-skinned fibres accurately describe events that occur in vivo given that the surface membrane is removed in this preparation? One of the most important features of mechanically-skinned fibres is that activation of Ca2+ release from the SR can be elicited by activation of the voltage-sensors (DHP receptors) present in the t-system just as it is in an intact fibre. That is, the normal E-C coupling mechanism is retained in this preparation. What is the evidence for this? The first indication came from experiments conducted by Natori in the fifties. He showed that small contractions could be elicited in mechanically-skinned fibres when large electrical stimuli (>110 V cm-1) were applied via electrodes to fibres under oil (Natori, 1954) (see later section). Later, Costantin & Podolsky (1967) showed that both electrical stimulation and raising the [Cl-] in the myoplasm of mechanically-skinned fibres produced contraction. At the time, not much was known about the mechanism of E-C coupling and these authors could only conclude that such contractions arose from depolarisation of some internal membrane compartment in the skinned fibre and favoured the idea that it involved both the t-system and SR. However, from this point in time electrical stimulation of skeletal muscle was not pursued further. Instead, the focus was directed towards the mechanism of Cl--induced activation of mechanically-skinned fibres. This led to the discovery that the t-system sealed off after skinning to form a separate compartment (see Fig 1). It was subsequently shown that by forming a separate compartment that is isolated from the myoplasmic environment of the fibre (as it is normally in an intact fibre), the t-system of a mechanically-skinned fibre could be polarized if the fibre was bathed in a solution that mimics the normal myoplasm (e.g.high [K+], some Na+, 8 mmol l-1 ATP, 10 mmol l-1 creatine phosphate, 1 mmol l-1 free Mg2+, 0.1 μmol l-1 Ca2+, pH 7.1) (Donaldson, 1985; Stephenson, 1985; Fill & Best, 1988; Lamb & Stephenson, 1990). Repolarization of the sealed t-system was possible due to the presence of functional Na+-K+ pumps that reestablish the normal Na+-K+ gradient (Donaldson, 1985; Stephenson, 1985; Fill & Best, 1988; Lamb & Stephenson, 1990). Some control of the t-system potential was then possible by simply changing the [K+] bathing the fibre (Fill & Best, 1988; Lamb & Stephenson, 1990; Posterino & Lamb, 1998a). If all the K+ in the bathing solution was rapidly removed, it was possible to depolarize the t-system. The K+ ion was often replaced with Na+, although in some instances, the K+ was replaced with choline chloride, in which the simultaneous increase in the [Cl-] helped to further depolarize the t-system (Lamb & Stephenson, 1990). Such depolarization led to the activation of force in mechanically-skinned fibres which was subsequently shown to involve the activation of both the DHPRs in the t-system and the RYR1s of the SR because such responses were: a) inhibited by antagonists of DHP receptors, such as nifedipine and verapamil (Posterino & Lamb, 1998a; Lamb &Stephenson, 1990); and b) completely blocked by ryanodine and ruthenium red, specific antagonists of RYRs (Lamb & Stephenson, 1990). The transient force responses observed following depolarization of the t-system in this manner last a few seconds and are graded by the myoplasmic [K+]. These results confirm that mechanically-skinned fibres retain functional E-C coupling and it is clear that such force responses are analogous to K+ contractures generated in intact fibres (i.e. when extracellular [K+] is increased). These results also showed that the essential elements involved in E-C coupling must be very robust as they are retained following mechanical-skinning and after the normal myoplasmic constituents are replaced with a minimal physiological solution (Lamb, 2000).
Nevertheless, despite many useful properties mentioned above, the mechanically-skinned fibre technique does have several limitations. One is that the t-system membrane potential can not be directly measured and importantly, can not be accurately controlled. A second, and perhaps the most important, is the slow depolarization of the t-system associated with diffusion of the bathing solution into the fibre. This prevents the study of rapid voltage-dependent Ca2+ release from the SR. Depolarization-induced force responses elicited by solution substitution occur with a rise time of some 500 ms, with the whole response lasting some 2-3 s. Consequently, force responses elicited in this manner may not be as sensitive to changes in Ca2+ release as a more rapid physiological response seen during a single twitch or a tetanus.
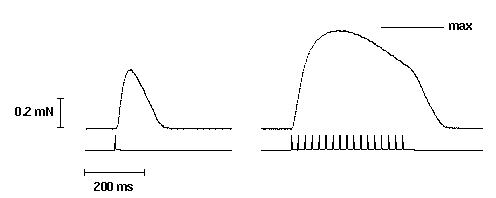
Figure 2. Twitch and tetanic (50 Hz) force responses elicited by applying 50 V cm-1
field stimulation (2 ms duration) to a segment of a mechanically-skinned EDL muscle fibre of the rat bathed in a solution mimicking the normal myoplasmic environment (ie. high [K+]; see Section 3). The applied
stimulus is simultaneous recorded below each force response. Maximum Ca2+-activated force (max)
was elicited in the same fibre using a heavily buffered Ca-EGTA solution and is indicated by the solid bar above the tetanic force response.
4. Electrical stimulation of mechanically-skinned fibres
As mentioned earlier, electrical stimulation of mechanically-skinned fibres has not been re-examined for some 25 years since the last experiments performed by Costantin. At the time large electrical stimuli were required to elicit relatively weak force responses. The reason for this may be due to the fact that mechanically-skinned fibres were stimulated under oil which left the t-tubules in a poorly polarised state. Furthermore, fibres were typically stimulated with a longitudinal electric field and with very large voltages that may have damaged the fibre. Recently, Posterino et al. (2000)revisited the idea of electrically-stimulating mechanically-skinned fibres, modifying both the solutions used to bath mechanically-skinned fibres and the orientation of the electric field. Thin platinum wire electrodes were positioned parallel with the long axis of the muscle fibre at a distance of 4 mm apart and along the whole length such that a uniform stimulus was applied. Fibres were bathed in a physiological solution that mimics the normal myoplasmic environment ensuring that the t-tubules were well polarized (Posterino & Lamb, 1998a; Lamb, 2000). A brief 2 ms, 20-25 V stimulus was applied giving a field strength of 50-60 V cm-1. In this manner, Posterino et al. (2000) were able to elicit reproducible twitch and tetanic force responses in mechanically-skinned fibres (see Fig 2). Precise positioning of the fibre between the electrodes was not necessary and twitch and tetanic force could be elicited in both mammalian fast-twitch fibres (Posterino et al., 2000) or amphibian twitch fibres (unpublished data).
Twitch or tetanic force responses in mechanically-skinned fibres are initiated by the generation of APs in the sealed t-tubules and by the activation of voltage-dependent processes that underlie normal E-C coupling (Posterino et al., 2000). Thus, even the first step in E-C coupling is retained in mechanically-skinned fibres – the ability to generate an AP. The evidence for this is threefold. Firstly, it was noted that the twitch response in fibres was steeply dependent on the applied voltage and exhibited a sharp threshold in which the transition between zero force and 70% of maximum twitch size required only a 10% increase in the applied electric field. Secondly, chronic depolarisation of the t-system prevented any twitch or tetanic response from being elicited by field stimulation. This is consistent with the inactivation of voltage-dependent processes that underlie both the AP and activation of the voltage-sensors of the t-system. And thirdly, the presence of 10 μM TTX in the sealed t-tubules strongly inhibited such responses proving that activation of Na+ channels (and the generation of an AP) in the t-system is essential in triggering further steps in the cascade.
The characteristics of both twitch and tetanic force responses elicited in mechanically-skinned fibres closely resemble the responses observed in intact fibres (Fryer & Neering, 1988; Schwaller etal., 1999). The peak amplitude of the twitch response in fast-twitch mammalian and twitch amphibian muscle is between 30% and 60% of maximum Ca2+-activated force. Tetani (50 Hz) elicits force responses between 80-100% of maximum Ca2+-activated force. The twitch-tetanus ratio in mammalian fast-twitch skinned fibres ranges between 0.40 and 0.60 which is larger than that observed in intact fibres (~0.30) (Schwaller et al., 1999). The larger twitch-tetanus ratio and the ability to achieve near maximal force during a tetanus, is possibly due at least in part to the loss of parvalbuminin mechanically-skinned fibre preparations (Stephenson et al., 1999).
The clear functional similarities between intact and mechanically-skinned fibres highlight the potential of the mechanically-skinned fibre technique in the study of skeletal muscle physiology. Nevertheless, there are a few differences in the responses observed between mechanically-skinned fibres and intact fibres. One such difference is that tetanic force in fast-twitch mechanically-skinned fibres declines much more rapidly during high frequency stimuli than in intact fibres, with force fading after ~200 ms of stimulation (termed fade; see Fig. 2). The cause of this phenomenon is not certain. Posterino et al. (2000) attributed this to a gradual build up of K+ in the sealed t-system with repeated APs leading to depolarization; this would not normally occur in an intact fibre where the t-system isopen to the extracellular environment. However, we have recently observed that this phenomena appears to be related to the fibre length, as fade was largely eliminated in fibres that were stretched from between 120% to 140% of their resting length (unpublished results). This is currently being further examined. Another difference between mechanically-skinned fibres and intact fibres is that the absence of a surface membrane means the intracellular Ca2+ content of the fibre can vary from the endogenous level. The comparatively large volume of solution bathing the mechanically-skinned fibre means that a substantial amount of Ca2+ can be gained from (or lost to) the bulk solution. In order to maintain the normal endogenous level of SR Ca2+, both over time and with repeated responses, it is necessary ensure that the free [Ca2+] of the bathing solution is buffered to the normal resting myoplasmic concentration (pCa 7.0). This limits the amount of Ca2+ loading. It is noteworthy that little variability is observed between successive twitch responses in mechanically-skinned fibres which suggests that the SR Ca2+ content remains relatively stable under the conditions used. However, a more precise determination of changes in the SR Ca2+ content during repeated stimulation is still needed as well as a better way of clamping the SR Ca2+ content.
5. Recent contributions and future directions
The ability to electrically stimulate mechanically-skinned fibres helps bridge the gap between biochemical and whole cell studies. Some recent findings illustrate the current and future potential of this technique towards the understanding of E-C coupling in skeletal muscle.
a) Mechanisms and pathways involved in the initial spread of excitation
The first step in E-C coupling involves the initiation and spread of the AP throughout the muscle fibre. It is generally accepted that the spread of excitation into the t-system in amphibian skeletal muscle involves an AP (Costantin, 1970; Bezanilla et al., 1972; Nakajima & Gilal, 1980). In mammalian skeletal muscle, it was not known if the spread was either passive or active, although it was assumed to involve an AP. As indicated earlier, the ability to generate an AP in mechanically-skinned fibres by electrical stimulation now provides direct evidence that excitation also spreads down the t-tubules via an active process in mammalian muscle (Posterino et al., 2000). This observation is unambiguous as there is no surface membrane present. Furthermore, it is apparent that other characteristics of the AP in the t-system can also be determined using this preparation. By using the twitch response as an indirect measure of the AP, it is possible to estimate the relative refractory period of the AP. If two single stimuli are elicited in close succession (less than 6 ms) no summation of the twitch force response was observed. However, if the second stimulus is applied 6 ms (or later) after the first, the twitch response is potentiated. This indicates that the refractory period of the AP is at most 6 ms long (unpublished data). Refinement of this measure could be achieved by examining the Ca2+ transient rather than force.
Experiments with electrically-stimulated mechanically-skinned fibres have also revealed the role of a previously identified structure in skeletal muscle. The observation that contractions (either spontaneous or elicited electrically) have the ability to propagate over hundreds of sarcomeres in mechanically-skinned fibres revealed a mechanism that allows excitation to spread throughout a skeletal muscle fibre independent of the surface membrane (Natori, 1954; Costantin & Podolsky, 1967; Posterino et al., 2000). It was found that an AP(s) could propagate along the entire length of skinned fibre segment (without the presence of a surface membrane) and could cause relatively synchronous activation of a large proportion of the fibre (>70%) travelling with an estimated velocity of some 13 mm s-1 (Posterino et al., 2000). It was suggested that the structure involved in the spread of the AP must be the longitudinal tubular system (LTS) which has been observed with electronmicroscopy (EM) (Franzini-Armstrong & Jorgenson, 1988; Stephenson & Lamb, 1992) and by confocal imaging of mechanically-skinned fibres in which a fluophore was trapped in the t-system(Peachey, L.D., 1965). These findings in mechanically-skinned fibres revealed a fundamental property that is likely to be important in the spread of the AP throughout a fibre, in fatigue and during myogenesis.
b) Internal transmission of excitation and control of Ca2+release
Data obtained from mechanically-skinned fibres in which functional E-C coupling is retained have also provided strong evidence for and against a number of ideas regarding the mechanism by which the DHPRs and the RYR1s communicate. It is clear from some experiments in mechanically-skinned fibres that the link between these two channels does not involve a diffusible second messenger such as inositol 1,4,5-trisphosphate (Walker et al., 1987; Posterino et al., 1998b) or Ca2+(Endo, 1985; Meissner et al., 1986; Lamb & Stephenson, 1991; Owen et al., 1997; Lamb & Laver, 1998). The link was also previously thought to involve the transient formation of disulphide bonds between the DHPRs and the RYRs (Salama et al., 1992). However, recent experiments in mechanically-skinned fibres have showed that strong sulphydryl reducing reagents do not interfere with normal E-C coupling(Posterino & Lamb, 1996). Nevertheless, various sulphydryl oxidants have been shown to modulate the RYR1 function (Dulhunty et al., 1996) indicating that the cellular redox state may effect E-C coupling in a more complex manner that is dependent on the precise ratio of endogenous redox reagents (ie. ratio of glutathione to reduced glutathione) both within the myoplasm and the lumen of the SR Feng et al., 2000). Here again we can see the potential advantage of mechanically-skinned fibres in addressing this question which can not readily be observed in an intact fibre preparation.
c) Ca2+handling
As mentioned earlier, mechanically-skinned fibres rapidly loose their parvalbumin after skinning and this may account for some of the dynamic differences between twitch responses observed between mechanically-skinned fibres and intact fibres. The absence of parvalbumin in mechanically-skinned fibres allows the examination of the functional role of this protein in SR Ca2+ handling. Most previous studies examining the role of parvalbumin have been limited by the inability to remove the effects of parvalbumin completely. Only one recent study using mice in which the parvalbumin gene has been knocked out has been able to examine the precise contribution of parvalbumin on twitch and tetanic characteristics (Fryer & Neering, 1988). This study showed that twitch-tetanus ratio was greater in fibres from parvalbumin knockout mice (PKM) than from wild type mice. Interestingly, the characteristics of the twitch response from PKM greatly resemble those from mechanically-skinned fibres. Nevertheless, it is possible that the absence of parvalbumin in PKM may have effected the other constituents important in E-C coupling and Ca2+ handling. The unique properties of mechanically-skinned fibres allows the examination of parvalbumin more precisely without the problem of non-specific effects that may arise from gene knockout. Parvalbumin can be simply addedto and removed from the bathing solution of mechanically-skinned fibres and the effects on the twitch and tetanus observed in the same fibre. Apart from parvalbumin, mechanically-skinned fibres can allow the precise examination of the role of the SR Ca2+ ATPase in contraction and relaxation. In intact fibre studies, the role of the SR Ca2+ ATPase is often examined by using specific inhibitors such as TBQ and thapsigargin (Westerblad & Allen, 1994; Caputo et al., 1999). However, it is difficult to be sure that complete block of the pump has taken place and that there are no complicating effects of increased resting Ca2+. Similarly, the intact fibre studies must also take into account any effects of parvalbumin. An advantage of mechanically-skinned fibres is that distinct qualities of the SR Ca2+ ATPase can be examined in isolation of parvalbumin and without changes to resting myoplasmic [Ca2+].
Conclusion
To date, mechanically-skinned fibres have been a useful tool in the study of many aspects of E-C coupling in skeletal muscle. The controlled nature of the myoplasmic environment of skinned fibres, the presence of functional E-C coupling that can be now be activated in the same manner and with a similar time course as an intact fibre, and the fact that the key structures involved in E-C coupling obviously remain as they were in vivo, demonstrate the potential of this technique in further aiding our understanding of E-C coupling in skeletal muscle.
Acknowledgments
I wish to thank Professor George Stephenson and Associate Professor Graham Lamb for their helpful advice and comments on the manuscript. This work was supported by the National Health and Medical Research Council of Australia.
References
Bezanilla, F., Caputo, C., Gonzalez-Serratos, H. & Venosa, R.A. (1972) Sodium dependence of the inward spread of activation in isolated twitch muscle fibres of the frog. Journal of Physiology, 223, 507-523.
Block, B.A., Imagawa, T., Campbell, K.P. & Franzini-Armstrong, C. (1988) Structural evidence for direct interaction between the molecular components of the transverse tubule/sarcoplasmic reticulum junction in skeletal muscle. Journal of Cell Biology, 107(6/2), 2587-2600.
Caputo, C., Bolanos, P. & Escobar, A.L. (1999) Fast calcium removal during single twitches in amphibian skeletal muscle fibres. Journal of Muscle Research & Cell Motility, 20, 555-567.
Costantin, L.L. & Podolsky, R.J. (1967) Depolarization of the internal membrane system in the activation of frog skeletal muscle. Journal of General Physiology, 50, 1101-1124.
Costantin, L.L. (1970) The role of sodium current in the radial spread of contraction in frog muscle fibres. Journal of General Physiology, 55, 703-715.
Donaldson, S.K.B. (1985) Peeled mammalian skeletal muscle fibres. Possible stimulation of Ca2+ release via a transverse tubule-sarcoplasmic reticulum mechanism. Journal of General Physiology, 86, 501-525.
Dulhunty, A.F. (1992) The voltage-activation of contraction in skeletal muscle. Progress in Biophysics and Physiology, 53, 1-16.
Dulhunty, A.F., Junankar, P.R., Eager, K.R., Ahern, G.P. & Laver, D.R. (1996) Ion channels in the sarcoplasmic reticulum of striated muscle. Acta Physiologica Scandanavica, 156, 375-385.
Endo, M. (1985) Calcium release from the sarcoplasmic reticulum. Current Topics in Membranes and Transport, 25, 181-230.
Favero, T.G. (1999) Sarcoplasmic reticulum Ca2+ release and muscle fatigue. Journal of Applied Physiology, 87(2), 471-483.
Feng, W., Guohua, L., Allen, P.D. & Pessah, I.N. (2000) Transmembrane redox sensor of ryanodine receptor complex. Journal of Biological Chemistry, 275, 35902-35907.
Fill, M.D. & Best, P.M. (1988) Contractile activation and recovery in skinned frog muscle stimulated by ionic substitution. American Journal of Physiology, 254, C107-114.
Franzini-Armstrong, C. & Jorgenson, A.O. (1988) Discrimination between fast and slow twitch fibres of guinea pig skeletal muscle using the relative surface density of junctional transverse tubule membrane. Journal of Muscle Research and Cellular Communication, 9, 403-413.
Franzini-Armstrong, C. & Protasi, F. (1997) Ryanodine receptors of striated muscles: a complex channel capable of multiple interactions. Physiological Reviews, 77, 699-729.
Fryer, M.F. & Neering, I.R. (1988) Actions of caffeine on fast- and slow-twitch muscles of the rat. Journal of Physiology, 416, 435-454.
Gordon, A.M., Homsher, E. & Regnier, M. (2000) Regulation of contraction in striated muscle. Physiological Reviews, 80(2), 853-924.
Konishi, M. & Watanabe, M. (1998) Steady state relation between cytoplasmic free Ca2+ concentration and force in intact frog skeletal muscle fibres. Journal of General Physiology, 111, 505-519.
Lamb, G.D. & Laver, D.R. (1998) Adaption, inactivation and inhibition in ryanodine receptors. In: The Structure and Function of Ryanodine Receptors. Ed. Sitsapesan, R. & Williams, A.J. Ch. 14. London: Imperial College Press.
Lamb, G.D. & Stephenson, D.G. (1990) Calcium release in skinned muscle fibres of the toad by transverse tubule depolarization or by direct stimulation. Journal of Physiology, 423, 495-517.
Lamb, G.D. & Stephenson, D.G. (1991) Effect of Mg2+ on the control of Ca2+ release in skeletal muscle fibres of the toad. Journal of Physiology, 434, 507-528.
Lamb, G.D. (2000) Excitation-contraction coupling in skeletal muscle: comparisons with cardiac muscle. Clinical and Experimental Physiology and Pharmacology, 27, 216-224.
Launikonis, B.S. & Stephenson, D.G. (1997) Effect of saponin treatment on the sarcoplasmic reticulum of rat, cane toad and crustacean (yabby) skeletal muscle. Journal of Physiology, 504.2, 425-437.
Launikonis, B.S. & Stephenson, D.G. (1999) Effects of β-escin and saponin on the transverse-tubular system and the sarcoplasmic reticulum membranes of rat and toad skeletal muscle. Pflügers Archiv, 437, 955-965.
Marx, S.O., Ondrias, K. & Marks, A.R. (1998) Coupled gating between individual skeletal muscle Ca2+ release channels (ryanodine receptors). Science, 281, 818-821.
Meissner, G. & Lu, X. (1995) Dihyropyridine receptor-ryanodine receptor interactions in skeletal muscle excitation-contraction coupling. Bioscience Reports, 15(5), 399-408.
Meissner, G., Darling, E. & Eveleth, J. (1986) Kinetics of rapid Ca2+ release by sarcoplasmic reticulum. Effects of Ca2+, Mg2+ and adenine nucleotides. Biochemistry, 25, 236-244.
Melzer, W., Herrmann-Frank, A. & Lüttgau, H.Ch. (1995) The role of Ca2+ ions in excitation-contraction coupling in skeletal muscle fibres. Biochimica et Biophysica Acta, 1241, 59-116.
Moisescu, D.G. (1976) Kinetics of reaction of calcium-activated skinned frog muscle fibres. Nature, 262, 610- 613.
Murray, B.E. & Ohlendiek, K. (1997) Cross-linking analysis of the ryanodine receptor and α1-dihydropyridine receptor in rabbit skeletal muscle triads. Biochemical Journal, 324, 689-696.
Nakai, J., Dirksen, R.T., Nguyen, H.T., Pessah, I.N., Beam, K.G. & Allen, P.D. (1996) Enhanced dihydropyridine receptor channel activity in the presence of ryanodine receptor. Nature, 380, 72-75.
Nakajima, S. & Gilal, A. (1980) Radial propagation of muscle action potentials along the tubular system examined by potential-sensitive dyes. Journal of General Physiology, 76, 751-762.
Natori, R. (1954) The property and contraction process of isolated myofibrils. Jikei-Kai Medical Journal, 1, 119-126.
Ogawa, Y. (1994) Role of ryanodine receptors. Critical Reviews in Biochemistry and Molecular Biology, 29(4), 229-274.
Ogawa, Y., Murayama, T. & Kurebayashi, N. (1999) Comparison of properties of Ca2+ release channels between rabbit and frog skeletal muscles. Molecular and Cellular Biochemistry, 190, 191-201.
Owen, V.J., Lamb, G.D., Stephenson, D.G. & Fryer, M.F. (1997) Relationship between depolarization-induced force responses and Ca2+ content in skeletal muscle fibres of rat and toad. Journal of Physiology, 498, 571-586.
Peachey, L.D. (1965) The sarcoplasmic reticulum and the transverse tubules of the frog's sartorius. Journal of Cell Biology, 25, 209-231.
Posterino, G.S. & Lamb, G.D. (1996) Effects of reducing agents and oxidants on excitation-contraction coupling in skeletal muscle fibres of rat and toad. Journal of Physiology, 496.3, 809-825.
Posterino, G.S. & Lamb, G.D. (1998a) Effect of nifedipine on depolarization-induced force responses in skinned skeletal muscle fibres of rat and toad. Journal of Muscle Research & Cell Motility, 19, 53-65.
Posterino, G.S. & Lamb, G.D. (1998b) Investigation of the effect of inositol trisphosphate in skinned skeletal muscle fibres with functional excitation-contraction coupling. Journal of Muscle Research & Cell Motility, 19, 67-74.
Posterino, G.S., Lamb, G.D. & Stephenson, D.G. (2000) Twitch and tetanic force responses and longitudinal propagation of action potentials in skinned skeletal muscle. Journal of Physiology, 527.1, 131-137.
Rall, J. (1996) The role of parvalbumin in skeletal muscle relaxation. News in Physiological Sciences, 11, 249- 255.
Rios, E. & Pizzaro, G. (1991) Voltage-sensor of excitation-contraction coupling in skeletal muscle. Physiological Reviews, 71, 849-908.
Salama, G., Abramson, J.J. & Pike, G.K. (1992) Sulphydryl reagents triggers Ca2+ release from the sarcoplasmic reticulum of skinned rabbit psoas fibres. Journal of Physiology, 454, 389-420.
Schneider, M.F. & Chandler, W.K. (1973) Voltage-dependent charge movement in skeletal muscle: a possible step in excitation-contraction coupling. Nature, 242, 244-246.
Schneider, M.F. (1994) Control of calcium release in functioning muscle fibres. Annual Review of Physiology, 56, 463-484.
Schwaller, B., Dick, J., Dhoot, G., Carroll, S., Vrbova, G., Nicotera, P., Pette, D., Wyss, A., Bluethmann, H., Hunziker, W. & Celio, M.R. (1999) Prolonged contraction relaxation cycle of fast-twitch muscles in parvalbumin knockout mice. American Journal of Physiology, 276, C395-403.
Sitsapesan, R. & Williams, A.J. (1997) Regulation of current flow through ryanodine receptors by luminal Ca2+. Journal of Membrane Biology, 159, 179-185.
Stephenson, D.G. & Lamb, G.D. (1992) Confocal imaging of key intracellular compartments in muscle fibres. Proceedings of the Australian Physiological and Pharmacological Society, 23, 244P.
Stephenson, D.G., Nguyen, L.T. & Stephenson, G.M.M. (1999) Glycogen content and excitation-contraction coupling in mechanically skinned muscle fibres of the cane toad. Journal of Physiology, 519(1), 177-187.
Stephenson, E.W. (1981) Activation of fast skeletal muscle: contributions of studies on skinned fibres. American Journal of Physiology, 240, C1-C19.
Stephenson, E.W. (1985) Excitation of skinned muscle fibres by imposed ion gradients. I. Stimulation of 45Ca efflux at constant [K][Cl] product. Journal of General Physiology, 86, 813-832.
Tanabe, T., Beam, K.G., Adams, B.A., Nicodome, T. & Numa, S. (1990) Regions of skeletal muscle dihydropyridine receptor critical for excitation-contraction coupling. Nature, 346, 567-569.
Walker, J.W., Somlyo, A.V., Goldman, Y.E., Somlyo, A.P. & Trentham, D.R. (1987) Kinetics of smooth and skeletal muscle activation by laser pulse photolysis of caged inositol 1,4,5-trisphosphate. Nature, 327, 249-252.
Westerblad, H. & Allen, D.G. (1994) The role of sarcoplasmic reticulum in relaxation of mouse muscle; effects of 2,5-di(tert-butyl)-1,4-benzohydroquinone. Journal of Physiology, 474.2, 201-301.
Yin, C.C. & Lai, F.A. (2000) Intrinsic lattice formation by the ryanodine receptor calcium-release channel. Nature Cell Biology, 2, 669-671.