Summary
1. The main aim of this review is to raise awareness of the molecular complexity of single skeletal muscle fibres from ‘normal’ and ‘transforming’ muscles, in recognition of the many types of hybrids that have been observed in vertebrate skeletal muscle. The data used to illustrate various points made in the review were taken from studies on mammalian (mostly rat) and amphibian muscles.
2. The review provides a brief overview of the pattern and extent of molecular heterogeneity in hybrid muscle fibres and of the methodological problems encountered when attempting to identify and characterise such fibres. Particular attention is given to four types of skeletal muscle hybrids: myosin heavy chain (MHC) hybrids, mismatched MHC-myosin light chains (MLC) hybrids, mismatched MHC-regulatory protein hybrids and hybrids containing mismatched MHC-sarcoplasmic reticulum protein isoforms.
3. Some of the current ideas regarding the functional significance, origin and cognitive value of hybrid fibres are critically examined.
Introduction
Skeletal muscle contraction is the net result of a series of cellular events known collectively as the excitation-contraction-relaxation (E-C-R) cycle. The major events in the E-C-R cycle were summarised by Stephenson et al (1998).
Major events in the E-C-R cycle in the vertebrate skeletal muscle include: (i) initiation and propagation of an action potential along the sarcolemma and transverse (T)-tubular system, (ii) transmission of the T-system depolarisation signal from the T-tubule to the sarcoplasmic reticulum (SR) membrane, (iii) release of calcium ions (Ca2+) from the SR, (iv) transient rise of myoplasmic [Ca2+], (v) binding of Ca2+ to the regulatory protein troponin C (Tn C), (vi) transient activation of the regulatory system and contractile apparatus, (vi) dissociation of Ca2+ from Tn C and (vii) Ca2+ reuptake by SR mediated by SERCA.
The key roles in these events are played by Ca2+ (the activation ion) and by a large number of proteins/protein complexes located in several subcellular compartments. It is now widely accepted that many of the proteins involved in events of the E-C-R cycle exist as multiple forms (isoforms), which can be distinguished and identified by biochemical methods such as gel electrophoresis and immunochemistry (Moss et al., 1995). Polymorphous skeletal muscle proteins of the E-C-R cycle include: the α -subunit of the dyhydropyridine receptor (DHPR), ryanodine receptor/SR calcium release channel (RyR), sarco (endoplasmic) reticulum Ca2+-ATPase (SERCA), calsequestrin, myosin heavy chain (MHC), myosin light chain (MLC) and the regulatory proteins troponin C (TnC), troponin I (TnI), troponin T (TnT) and tropomyosin (Tm) (Pette & Staron, 2000, 1997, 1990). It is important to point out that the list of isoforms of skeletal muscle proteins (particularly myofibrillar proteins) has been increasing in parallel with the development/refinement of protein separation/identification techniques and with the application of these techniques to a wider range of muscles and animal species. Updated versions of this list can be found in reviews produced regularly by major contributors to the field ( Pette & Staron, 2000; Schiaffino & Salviati, 1998; Pette & Staron, 1997; Schiaffino & Reggiani, 1996; Pette & Staron, 1990; Swynghedauw, 1986).
It is noteworthy that the number of isoforms varies markedly between various skeletal muscle proteins. For example, according to a recent count, mammalian skeletal muscle expresses as many as ten MHC isoforms, but only two TnC isoforms [one typical of fast-twitch muscle (TnC-f), the other of slow-twitch muscle (TnC-s) (Pette & Staron, 2000; 1997). The ten MHC isoforms* include four isoforms present in adult mammalian muscle (slow-twitch isoform MHCI or MHCβ/slow and fast-twitch isoforms MHCIIa, MHCIId/x and MHCIIb), two isoforms present in developing and regenerating muscles (MHC-emb, MHC-neo), and four isoforms present in some highly specialized muscles [extraocular and jaw closing muscles (MHC-exoc). Currently it is not known whether there is any relationship between the number of isoforms of a muscle protein, its cellular function and/or the molecular mechanisms responsible for its molecular diversity.
So far, the terms ‘hybrid muscle fibres† ’, ‘polymorphic fibres’, or ‘MHC hybrids’ have been used interchangeably to define fibres that co-express more than one MHC isoform. However, there is now compelling evidence to suggest that this meaning of the term ‘hybrid fibres’ is highly inadequate because it does not apply to fibres displaying patterns of molecular heterogeneity with respect to other proteins. A population of such fibres, expressing only one MHC isoform (MHCIIa) and both fast- and slow-twitch isoforms of the MLC subunits has been detected in rat soleus (SOL) in an early study by Mizusawa et al. (1982) and in a very recent study by Bortolotto et al. (2000a). Based only on MHC composition, these fibres would be classified as ‘pure’, but such classification would be incorrect because it would not provide information on the molecular heterogeneity of the fibres with respect to MLC composition. Two other major groups of hybrids not covered by the traditional meaning of the term ‘hybrid fibres’ include fibres in which two or several proteins are expressed as isoforms (e.g. fibres containing several isoforms of MLC and several isoforms of tropomyosin) and fibres in which one isoform is expressed as a protein and another as a mRNA species. It is important to note that the sets of isoforms detected so far in fibres co-expressing isoforms of two or more muscle proteins have been found to be either of the same type (matched) or of different types (mismatched). Matched sets of MHC and MLC isoforms would comprise, for example, fast-twitch MHC isoforms MHCIIa and MLCIIb and fast-twitch MLC isoforms MLC1f , MLC2f and MLC3, while mismatched sets would comprise fast-twitch MHC isoforms MHCIIa and MHCIIb, slow-twitch MLC isoform MLC1s and fast-twitch MLC isoforms MLC1f, MLC2f and MLC3. In recognition of the many kinds of hybrids that have been observed so far in vertebrate skeletal muscles, the hybrid fibres discussed in this review will be described by terms that indicate both the muscle protein(s) whose isoforms are being considered (e.g. MHC-MLC ) and the relationship (matched or mismatched) between the sets of isoforms co-expressed in the fibre.
It is worth pointing out that hybrid fibres were once regarded as a rare phenomenon and often discarded from studies concerned with the functional characteristics of single fibre preparations (Danieli-Betto et al., 1990). More recently, however, hybrid muscle fibres have started to attract considerable interest from a broad range of cell biologists. This can be explained, in part, by the finding that MHC hybrids represent the dominant biochemical phenotype even in skeletal muscles that were previously thought to be ‘pure’ in terms of fibre type composition (Bortolotto et al., 2000a).
This is the first review to focus on skeletal muscle hybrid fibres. Its main aim is to raise awareness of the molecular complexity of single muscle fibres, particularly among physiologists concerned with basic and applied aspects of skeletal muscle function. The data used to illustrate various points made in the review were taken from studies of mammalian (mostly rat) and amphibian muscle. The general background sections provide a brief overview of the pattern and extent of molecular heterogeneity in hybrid muscle fibres from ‘normal’ and ‘transforming’ muscles and of the methodological problems encountered when attempting to identify and characterise such fibres. Particular attention is given to four types of skeletal muscle hybrids: MHC hybrids, mismatched MHC-MLC hybrids, mismatched MHC-regulatory protein hybrids and mismatched MHC-SR protein hybrids. The last section of the review comprises a critical examination of some of the current ideas regarding the functional significance, origin and cognitive value of hybrid fibres.
MHC Hybrids
Methods used for the detection and characterisation of MHC hybrids.
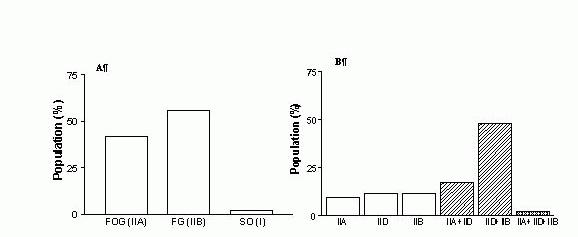
It is now quite clear that neither of the three methods traditionally employed to distinguish fibre types in skeletal muscles [light microscopy, myosin/myofibrillar ATPase (mATPase)-based or metabolic enzyme-based histochemistry] can be used effectively for the identification and characterisation of MHC hybrids (Hämäläinen & Pette, 1995; Schiaffino & Reggiani, 1996). The most suitable methods for detecting MHC polymorphism in individual muscle fibres, at protein or mRNA level, include MHC-based immunohistochemistry (MHC- IHChem), single fibre polyacrylamide gel microelectrophoresis under denaturing conditions (SDS-PAGEsf), pyrophosphate gel electrophoresis of myosin isoenzymes in single fibre segments, reverse transcription-polymerase chain reaction (RT-PCR) and in situ hybridisation (for review see Pette & Staron, 2000; Pette et al., 1999; Schiaffino & Salviati, 1998; Hämäläinen & Pette, 1995). This methodological point is well illustrated by the bar graph shown in Figure 1, which allows a quick comparison of the fibre type composition of rat extensor digitorum longus (EDL) (A,B) and SOL (C,D) muscles reported by Armstrong and Phelps (1984), on the basis of mATPase-based histochemistry (A, C) and that found in more recent studies using SDS-PAGEsf only (EDL; Bortolotto et al., 2000a) (B) or a combination of SDS-PAGEsf and MHC-IHChem (SOL, Bottinelli et al., 1994a) (D). A significant conclusion emerging from these data is that the largest proportion (~70%) of fibres in rat EDL are hybrid IIB+IID fibres, rather than IIA or IIB fibres, as previously thought. The newly discovered heterogeneity of rat EDL muscle and muscle fibres has important theoretical and methodological implications because rat EDL has been for many years the preferred experimental model in physiological investigations of mammalian fast-twitch muscle contractility.
It is important to note that even methods such as MHC-based IHChem and SDS-PAGEsf have intrinsic limitations when used for the detection and characterisation of MHC hybrids. Some of these limitations have been pointed out by Pette et al.(1999) and Schiaffino & Salviati (1998). For example, MHC-based IHChem is limited by the availability and specificity of anti-MHC isoform antibodies and it does not always detect fibres containing both MHCIId and MHCIIb isoforms (IID + IIB hybrids; Rivero et al., 1998). Furthermore, while MHC-based IHChem can provide considerable information about the proportion and intramuscular distribution of hybrid fibres and about the intracellular distribution of the co-expressed MHC isoforms (Dix & Eisenberg, 1988), it does not allow for the quantification of MHC isoforms co-expressed in individual hybrid fibres. By comparison, SDS-PAGEsf combined with scanning densitometry, enables the researcher to separate and quantify the relative proportion of MHC isoforms co-expressed in a single fibre, and to relate these results to other physiological or biochemical parameters determined in the fibre prior to incubation in the sample solubilising buffer. However, a notable limitation of SDS-PAGEsf is that it does not provide information on the intramuscular distribution of hybrid fibres. Also, it has to be stressed that when applied to MHC isoform analyses, SDS-PAGEsf displays a high degree of variability with respect to the effectiveness of separation of MHC isoform bands, a problem which has not been eliminated despite intense efforts made in several laboratories. In practice, this means that that each fibre sample has to be electrophoresed, at the same time with a MHC isoform marker (a reference sample containing all MHC isoforms), on several gels, until the separation of MHC isoform bands allows meaningful densitometric analyses to be carried out. Moreover, a SDS-PAGEsf protocol that separates well MHC isoforms in one species may not be so effective when applied to another species (Nguyen & Stephenson, 1999). The low specificity of anti-MHC isoform antibodies and the difficulties related to the electrophoretic resolution of MHC isoform bands are probably due to the high degree of molecular homology (78-98% aminoacid identity in striated muscles; Weiss & Leinwand, 1996) displayed by MHC isoforms and therefore cannot be eliminated in a simple manner.


Figure 1. The fibre type composition of rat EDL (A,B) and SOL (C,D) muscles, as determined by mATPase histochemistry (A, C) and single fibre SDS-PAGE (B, D). The graphs were plotted using the data reported by Armstrong & Phelps (1984) (A, C), Bortolotto et al. (2000a) (B) and Botinelli et al. (1994) (D). FOG, fast oxidative glycolytic; FG, fast oxidative; SO, slow oxidative.
Since many functional parameters of a hybrid fibre are not tightly correlated to its MHC isoform expression, neither MHC-based IHChem nor SDS-PAGEsf analyses of MHC isoform composition can accurately predict/characterise the functional phenotype of hybrid fibres (e.g. Bortolotto et al., 2000a). Thus, in order to gain further insights into the structural and functional complexity of MHC hybrids, one needs to combine creatively existing microanalytical, microphysiological and microhistochemical methods and/or develop new methods for single fibre analysis (Pette et al., 1999). For example, by applying three different biochemical methods (in situ hybridization, MHC-based IHChem and mATPase histochemistry) to serial cryosections from human vastus lateralis muscles (prior and post-37 day period of bed rest), Andersen et al. (1999) discovered a novel population of MHC hybrids, in which the protein of one MHC isoform (MHCI) coexisted with the mRNA of another (MHCIId). The discovery of MHC hybrids with mismatched protein-mRNA species adds a new meaning to the concept of skeletal muscle cell heterogeneity and prompts the obvious question whether vertebrate skeletal muscles contain any fibres that are genuinely ‘pure’.
Table I.
Evidence that coexistence of multiple
MHC
isoforms in a single fibre is a common
motif across a broad spectrum of normal vertebrate skeletal muscles and that the
pattern of
MHC
polymorphism is muscle and animal species specific.
| Muscle† | Species | Proportion of hybrids | Number of MHC isoforms detected | Pattern of MHC isoform co-expression in hybrid fibres | Reference [method used†] |
|
EDL
DPH |
rat |
67%
30% |
2 or 3
2 or 3 |
IIa+IId; IId+IIb (majority); IIa+IId+IIb
I+IIa; I+IId; IIa+IId; IIa+IId+IIb; I+IIa+IId |
Bortolotto et al., (2000) [a] |
|
Plantaris
TA-superficial SOL |
rat |
~50%
~30% 11% |
2 or 3
2 2 |
IIa+IId; IIb+IId (majority); IIa+IIb+IId
IId+IIb I+IIa |
Bottinelli et al., (1994a) [a,b] |
| MG | rat | 12% | 2 | I+IIa; IIa+IId (majority); IIb+IId | Rivero et al., (1998) [b] |
| PCA* | rat | ~35% | 2, 3, or 4 | IId+IIb (majority); IIIb+exoc; I+IIa+IId; IIa+IId+IIb; IIa+IIb+exoc; IId+IIb+exoc; I+IIa+IId+IIb; IIa+IId+IIb+exoc | Wu et al., (2000a) [a] |
| ThA* | rat | ~90% | 2 or 3 | IId+IIb; IIb+exoc; IId+IIb+exoc | |
| AM | rabbit | not available | 2 | IIb+IId; IId+IIa; IIa+I | Aigner et al., 1993 (mATPase & SDS-PAGEsf) |
| LC* | dog | 41% | 2 or 3 | IIa+IId; I+IIa; I+IIa+IId | Wu et al., 1998 (SDS-PAGEsf) |
| PCA* | dog | ~20% | 2, 3 or 4 | IIa+IId (majority); IIa+IIb; IId+IIb;I+IIa+IId; IIa+IId+IIb; I+IIa+IId+IIb | Wu et al., 2000b (SDS-PAGEsf) |
| CT* | <10% | 2, 3 or 4 | I+IIa; IIa+IId; IId+IIb;I+IIa+IId+IIb | ||
| ThA* | 30-40% | 2, 3 or 4 | IIa+IId and IId+IIb (majority); I+IIa+IId;I+IId+IIb; IIa+IId+IIb; I+IIa+IId+IIb | ||
| Rectus abdominis | cane toad | ~65% | 2 or 3 | HCT+HC1 ; HCT+HC3 ; HC1+HC2 ; HC2+HC3 ; HCT+HC1+HC3 ; HCT+HC2+HC3 ; HC1+HC2+HC3 | Nguyen & Stephenson, (in preparation) [a]† |
MHC hybrids detected in ‘normal’* muscles
Fibres co-expressing the slow-twitch and fast-twitch MHC isoforms MHCI and MHCIIa (I+IIA or I/IIA fibres) are the earliest examples of MHC hybrids reported to occur in normal mammalian skeletal muscle. These fibres, previously referred to as IC or IIC fibres on the basis of the most abundant MHC isoform expressed (MHCI or MHCIIa, respectively) (Pierobon-Bormioli et al., 1981), are still the easiest to identify and characterise both by MHC-based IHChem and SDS-PAGEsf. This is because the currently available monoclonal antibodies (Mabs) against MHCI and MHCIIa have a high degree of specificity and the electrophoretic bands corresponding to the MHCI and MHCIIa proteins are separated quite effectively and reproducibly by all SDS-PAGEsf protocols developed and/or used in different laboratories. As described in detail in a recent study by Bortolotto et al. (2000a), type I+IIA hybrid fibres can also be identified by the physiological fibre typing method of Fink et al. (1986), because they produce characteristic staircase-like force-pSr curves (‘composite’ curves).
A large number of data generated over the last two decades by MHC-based IHChem and/or SDS-PAGEsf, strongly suggest that normal muscles from mammals and amphibians contain a sizeable proportion of MHC hybrids, which co-express, at the protein level, two, three and even four MHC isoforms. A small sample of these data (Table I) shows that the pattern of MHC polymorphism (as indicated by the proportion of hybrids, the number of isoforms co-expressed and the combination of MHC isoforms detected in individual fibres) is muscle and animal species specific. For example in adult rat, soleus muscle (SOL) was found to contain about 11% MHC hybrids, all of which co-expressed two MHC isoforms (I and IIa), while laryngeal thyroarytenoid (ThA) muscle was found to contain about 90% MHC hybrids, some of which co-expressed combinations of two or even three MHC isoforms. In the dog, however, ThA muscle was found to contain a smaller proportion (30-40%) of MHC hybrids and many of these hybrids co-expressed all 4 major MHC isoforms commonly found in mammalian muscle (I, IIa, IId and IIb). MHCexoc, a tissue specific isoform co-expressed with MHCIId and MHCIIb in rat ThA muscle was not detected in dog ThA muscle.
MHC hybrids detected in muscles in transformation
It is now widely accepted that the proportion of MHC hybrids and their molecular complexity (as judged by the number of MHC isoforms co-expressed and the pattern of co-expression) is higher in muscles undergoing molecular and functional transformation than in normal muscles (see review by Pette et al., 1999). As seen in Table II, soleus muscles of rats subjected to four week unloading by hindlimb suspension (a strategy inducing a slow to fast transition in muscle phenotype) contained 4 times more hybrid fibres than the controls (Oishi et al., 1998). Moreover, most hybrid fibres in the transforming soleus co-expressed three MHC isoforms (I, IIa and IId) , while all hybrids in the control soleus co-expressed two MHC isoforms only (I and IIa). Some MHC isoform combinations, such as I+ IId, which are seen only rarely in fibres from normal muscles, have been found to occur fairly frequently in fibres from transforming muscles (Pette & Staron, 2000, 1997; Talmadge, 2000). For example, Bortolotto et al. (2000a) detected only two I + IID hybrids in a population of 43 fibres dissected from 8 normal rat diaphragms, but I + IID hybrids were reported to make up the most abundant fibre-type population in SOL muscles of adult rat, 60 days post spinal cord transection (see Table II; Grossman et al., 1998).
TABLE II. EXAMPLES OF MHC
POLYMORPHISM ASSOCIATED WITH EXPERIMENTALLY INDUCED MUSCLE
TRANSFORMATION
|
Muscle (method used to induce muscle transition) |
Species |
Proportion of hybrids-experimental (proportion of hybrids-control) |
Number of MHC isoforms detected in hybrids (vs control) |
Pattern of MHC isoform co-expression in hybrid fibres (vs control) |
Reference (method†
used) |
| slow→fast | |||||
| SOL (4 wk-unloading by HS) | rat | 32% (7%) | 2 or 3 (2) | I+IIa; IIa+IId; I+IIa+IId*; IIa+IId+IIb (I+IIa) | Oishi et al., (1998) [a] |
| SOL (60 days post SCT) | rat | ~88% (~4%) | 2 or 3 (2) | I+IIa; I+IId; IIa+IId; I+IIa+IId (I+IIa) | Grossman et al., (1998) [b] |
| SOL (4 wk- thyroid hormone treatment) |
rat (male)
rat (female) |
99% (ni)
63% (ni) |
2 & 3
2 & 3 |
I+IIa; I+IIa+IId (ni)
I+IIa; IIa+IId; αcl+IIa; I+IIa+IId (ni) |
Yu et al., (1998) [b] |
| fast →faster | |||||
| MG-deep region (60 days post SCT) | rat | ~55% (~15%) |
2,3 or 4
(2) |
I+IIa; I+IId; IIa+IId; IId+IIb;
IIa+IId+IIb; I+IIa+IId; I+IIa+IId+IIb (I+IIa; IIa+IId; IId+IIb) |
Roy et al., (2000) [b] |
| MG-superficial region (60 days post SCT) | rat | ~7% (~22%) |
2 or 3
(2 or 3) |
IIa+IId; IId+IIb; IIa+IIb+IId
(IIa+IId; IIa+IId+IIb) |
ditto |
| fast→slow | |||||
| EDL (28 d low frequency stimulation) | rat | ? | 2 and 4 (2) |
I+IIa; IIa+IId; I+IIa+IId+IIb
(IIb+IId) |
Termin et al., (1989) [a,c] |
| TA (30 d low frequency stimulation) | rabbit | 70% (ni) | 2 or 3 (ni) | IIa+Iα; IIa+Iα+dev; IIa+Iα+I; IIa+I; Iα+I; Iα+I+dev;I+dev (ni) | Peuker et al., (1999) [b] |
The transition of a muscle from one ‘steady-state’ to another, through a process which may involve the replacement or addition of muscle protein isoforms in one or several intracellular compartments, has been found to accompany several physiopathological conditions/factors (Table III). These include growth and development of an organism from embryonic to adult stage, ageing, muscle degeneration/regeneration and changes in the hormonal level (thyroid hormone being the classical example) (Pette & Staron, 2000, 1997). For example, La Framboise et al. (1991) reported that before reaching the adult state, the rat diaphragm muscle contained some fibres that co-expressed as many as four MHC isoforms.
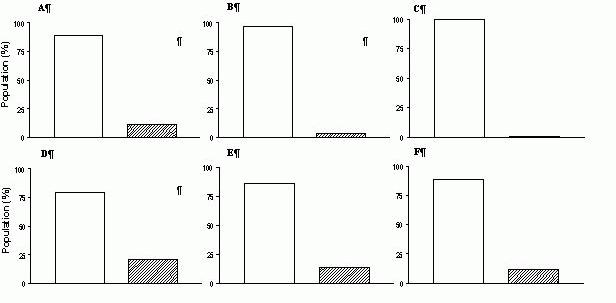
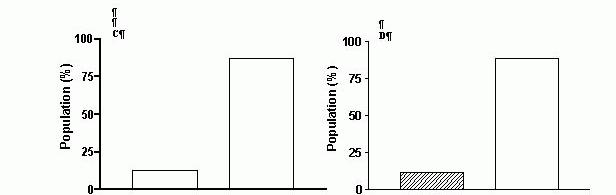
Figure 2.
The proportion of pure and hybrid fibres in soleus muscles from age-matched normotensive
WKY rats (A,B,C) and spontaneously hypertensive rats (D,E,F) at three stages of development of hypertension: 4 weeks (A,D), 16 weeks (B,E) and 24 weeks (C,F). , pure fibres (I, IIA) , hybrid fibres: I+IIA (in WKY rats) I+IIA; IIA+IID (in SHRs). The raw data used to plot the bar graphs can be found in Table 3 in Bortolotto et al. (1999). The number of animals used and the number of fibres analysed for each group were : 7; 73 (A); 8; 85 (B); 7; 79 (C); 7; 75 (D); 6; 73 (E); 7; 87 (F).
Recently, Bortolotto et al. (1999) observed, using SDS-PAGEsf, that the populations of fibres dissected from SOL muscles of spontaneously hypertensive rats (SHR) at three different stages in the development of hypertension (4, 16-18 and 24 weeks) contained a higher proportion of hybrid fibres than the homologous muscles from age matched normotensive (WKY) controls (Fig.2). Does this mean that hypertension, a pathological condition not generally associated with skeletal muscle pathology, causes transformation in rat soleus muscles? Not necessarily, because the higher proportion of MHC hybrids found in SHR soleus may be due to strain-related differences in the fibre type composition of individual muscles. To address this possibility we are now examining the MHC isoform expression in soleus muscles from several normotensive and hypertensive rat strains (J. Kemp, S. Bortolotto & G. M.M. Stephenson, unpublished data).
|
Factor/ condition |
Direction of phenotype shift |
| • muscle growth and development to neonatal stage | embryonic → neonatal |
| • maturation and ageing | fast → slow |
| • spontaneous hypertension (model for hypertension) | slow → fast |
| • thyroid hormone administration (hyperthyroidism) | slow → fast |
| • muscle degeneration | |
|
•
muscle regeneration
- post myotoxic treatment - post denervation-devascularization |
|
| • lab induced-decrease in neuromuscular activity | |
| - spinal cord transection (model for : spinal cord injury, orthopedic injury) | slow → fast |
| - limb immobilization in a shortened position | slow → fast |
| - hindlimb suspension (model for weightlessness during spaceflight) | slow → fast |
| - blockage of motoneuron action potential conduction by tetrodotoxin | fast → slow |
|
•
lab-induced increase in neuromuscular activity
- chronic electrical stimulation |
fast → slow |
| - functional overload induced by synergist ablation | |
| - endurance training | fast → slow |
| - strength (resistance) training | fast → slow |
| • lab induced depletion in energy rich phosphates | |
| • induction of null mutations in muscle protein isoform genes |
The main aim of the study by Bortolotto et al. (1999) was to compare the MHC isoform and fibre type composition of soleus muscles from SHR and WKY rats; however, since the study involved the use of animals at three different developmental stages, the data generated offer also an insight into the effect of rat maturation on the proportion of hybrid fibres present in this muscle. As seen in Figure 2, the proportion of hybrids detected in the fibre populations dissected from SOL muscles of WKY rats aged 4 weeks, 16 weeks and 24 weeks decreased from 11% (4 weeks) to 3.5% (16 weeks) and 0% (24 weeks). A decrease in the proportion of hybrid fibres with an increase in animal age was also noted in the soleus muscles from SHRs. In contrast, the rectus abdominis muscles of ‘adult’cane toads (bw ~250 g) produced a larger proportion of MHC hybrids than the homologous muscles from ‘juvenile’ (bw ~15g) toads (L. Nguyen & G.M.M. Stephenson, unpublished data). This difference in the effect of animal maturation on the proportion of hybrid fibres present in rat soleus and toad rectus abdominis muscle suggests that muscle transformation associated with development may be animal species- and/or muscle-specific.
A limited survey of the literature on vertebrate skeletal muscle reveals that, over the last two decades, there has been a flurry of activity in the field of muscle transformation. This, together with the breadth of journals that published this information (for recent reviews see Pette & Staron, 2000; Talmadge, 2000; Pette & Staron, 1997), indicate that, muscle plasticity, like muscle heterogeneity, is currently viewed as a ‘hot’ research topic by biomedical researchers from a broad range of disciplines (molecular biology, biochemistry, cell physiology, medical practice and exercise physiology).
Other Types of Hybrid Fibres Detected in Vertebrate Skeletal Muscles
Methodological issues
It is important to point out that highly complex hybrid fibres, such as those containing matched or mismatched sets of isoforms for two or more proteins, are more difficult to detect than MHC hybrids, which are heterogeneous with respect to MHC isoform composition only. This is because many muscle proteins, such as the proteins of the sarcotubular system, are present in the fibre only in very small amounts and therefore cannot be easily visualised on SDS-polyacrylamide gels using current staining protocols. Even when the concentration of a given protein in a single fibre segment is large enough to allow for easy visualisation, as is the case for most myofibrillar proteins, there may be problems related to the electrophoretic separation of the protein bands of interest (see comments made earlier regarding the separation of MHC isoforms). A classic example is that of the fast-twitch and slow twitch isoforms of MLCs, troponin subunits and tropomyosin, which either co-migrate or migrate very closely on SDS-polyacrylamide gels prepared according to common protocols. In such cases, the accurate identification of hybrid fibres requires further refinement of SDS-PAGEsf protocols, the combined use of SDS-PAGEsf and IHChem or the combined use of several biochemical and physiological methods of single fibre analysis. The following sub-sections focus on three groups of hybrid fibres co-expressing mismatched sets of protein isoforms and highlight, when appropriate, the methodological approaches that led to their discovery.
Mismatched MHC-MLC hybrids
Single muscle fibres that express only one MHC isoform, but are heterogeneous with respect to their myosin light chain complement represent a relatively common example of MHC-MLC polymorphism (for review see Pette & Staron, 1997). Thus, a small number of mismatched MHC-MLC hybrids have been detected among fibres dissected from diaphragm muscles of adult normotensive (WKY) rats (Bortolotto et al., 2000a), indicating that mixed expression of MHC and MLC isoforms occurs in normal muscle fibres. In Figure 3 are shown the electrophoretograms of two of the fibres analysed by Bortolotto et al. (2000a) for MHC and MLC composition: one displaying full correlation (MHCI + MLC1s + MLC2s; left lane) and the other no correlation between the myosin subunit isoforms present in the fibre (MHCIIa + MHCIId + MLC1s + MLC1f + MLC2f; right lane). Another interesting observation made in the study of Bortolotto et al. (2000a) is that, in the rat soleus muscle, fibres expressing only fast MHCIIa isoforms contained the slow isoform MLC1s in addition to the fast isoforms MLC1f and MLC2f. This result is in agreement with earlier data by Mizusawa et al. (1982) and Salviati et al. (1982) who showed also that type IIA fibres isolated from soleus muscles of the rat (Mizuzawa) and rabbit (Salviati) co-expressed various combinations of fast and slow MLC isoforms.
Mismatched MHC-MLC hybrids have been detected not only in normal, but also in transforming muscles. For instance, ‘pure’ slow-twitch (type I) soleus fibres from female rats, treated for 4 weeks with the thyroid hormone (T3), were found by Yu et al. (1998) to contain both slow and fast MLC isoforms, in different combinations and in varying proportions.
Notwithstanding their presence in many of the commonly studied muscles and the relative ease with which they are detected by SDS-PAGEsf
, the physiological significance of MHC-MLC hybrids
remains largely unknown.
Mismatched MHC-regulatory protein hybrids
To date, reports of mixed MHC-regulatory protein hybrids are few and far between. For example two decades ago, Salviati et al. (1982) described a small population of rabbit masseter muscle fibres, which contained fast-twitch and slow-twitch isoforms of MHCs, MLCs, TnT and Tn I, but only the fast-twitch isoform of TnC. Mismatched MHC-TnC hybrids co-expressing either MHCI (slow), TnC-s (slow) and TnC-f (fast) or MHCI and TnC-f were also detected in rat diaphragm muscle by Danieli-Betto et al. (1990) and Geiger et al. (1999), respectively. Since adult normal animals were used in all three studies, these data suggest that single fibres from normal muscles co-express mismatched sets of MHC and TnC isoforms.
At present it is not clear whether mismatched MHC-regulatory protein hybrids occur also in transforming skeletal muscles. Kischel et al. (2001) did not detect any fibres containing mismatched MHC and TnC isoforms in rat soleus muscles undergoing a shift from slow- to fast-twitch phenotype, but in this study the MHC isoform composition was ‘deduced’ from MLC isoform composition rather than determined directly. Given that in rat skeletal muscle fibres there is no tight correlation between MLC and MHC isoform expression (see previous section), it is possible that the MHC isoform composition in some single fibres was not correctly assessed and therefore mismatched MHC-TnC hybrids were overlooked.
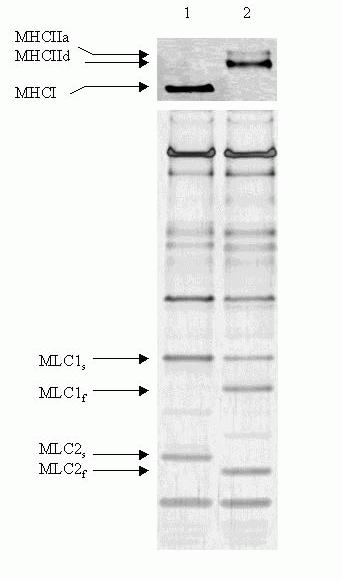
Figure 3. Representative electrophoretograms of myofibrillar proteins from single rat skeletal muscle showing matched (lane 1) and mismatched (lane 2) MHC-MLC isoform composition. Upper panel: MHC isoforms; lower panel: MLC isoforms. Lane 1, fibre type I; Lane 2, fibre type IIA + IID.
As mentioned previously, the visualisation and identification of Tn subunit isoforms on SDS polyacrylamide gels are fraught with problems, because the electrophoretic bands are not well separated from each other and from MLC2 isoform bands. This point is well illustrated in the study of Kischel et al. (2001), because the authors combined three methods in order to positively identify the TnC isoforms expressed in their fibre preparations: SDS-PAGEsf , Western Blotting and measurements of the sensitivity of chemically skinned fibre segments to activation by Sr2+ and Ca2+ (in the presence/absence of the fibre-type dependent Ca2+ sensitiser molecule bepridil).
Mismatched MHC - SR protein hybrids
According to the current dogma, SR protein complexes such as the Ca2+ release channel/ryanodine receptor (RyR) and SERCA play key roles in skeletal muscle contraction and relaxation by regulating the concentration of activating ions ([Ca2+]) in the myoplasm (see review by Stephenson et al., 1998). Mammalian skeletal muscles have been shown to express two different RyR isoforms (predominantly RyR 1 and some RyR 3; Csernoch, 1999) and three different SERCA isoforms (SERCA 1a, SERCA 1b and SERCA 2a; Loukianov et al., 1998). SERCA 1a is present in typical fast-twitch fibres and SERCA 2a is present in typical slow-twitch fibres.
There are very few reports of mixed MHC - SR protein hybrids in the literature concerned with skeletal muscle heterogeneity. For example, in a paper on the fibre-specific regulation of Ca2+-ATPase isoform expression by thyroid hormone in rat skeletal muscle, the authors (Van der Linden et al., 1996) describe a small population of fast-twitch fibres, dissected from soleus muscles of euthyroid (‘normal’ muscles) and hypothyroid rats (muscles ‘in transition’), which expressed the fast-twitch isoform MHC IIa and both fast-twitch SERCA1a and slow-twitch SERCA2a isoforms. Other examples of mixed MHC-SR protein hybrids can be found in a recent study by Bortolotto et al. (2001), who detected in soleus muscles of spontaneously hypertensive rats (SHR) a population of type I (slow-twitch) fibres displaying fast-type SR characteristics, and another population of type II (fast-twitch) fibres displaying slow-type SR characteristics (see Fig. 4).
Once again it is interesting to note the methodological approaches that allowed the identification of the mismatch between the protein isoform composition of the myofibrillar compartment and that of the SR. The MHC-SERCA hybrids described in the study of Van der Linden et al. (1996) were identified by IHChem with a protocol using fluorescence labelled antibodies against MHCI, MHCII, SERCA1a and SERCA2a, while the MHC-SR protein hybrids described in the study of Bortolotto et al. (2001), were identified by a combination of biochemical (SDS-PAGEsf) and physiological methods (measurements of caffeine thresholds for contraction in mechanically skinned single fibre preparations).
Functional Significance, Origin and Experimental Value of Hybrid Skeletal Muscle Fibres
Functional significance of hybrid fibres
As it has been already discussed, hybrid fibres exist in both transforming and normal skeletal muscles, and in some muscles they represent the predominant phenotype. What is unclear, however, is whether hybrid fibres play a major role in the mechanical performance of a muscle or whether they are incompletely differentiated muscle cells, and as such, functionally redundant entities. The issue of the functional significance of hybrid fibres is further complicated by compelling evidence that some hybrid fibres are persistent rather than transitory cellular species (Lutz & Lieber, 2000; Bortolotto et al., 2000a; Talmadge, 2000; Talmadge et al., 1999)
At present, the prevailing view is that hybrid fibres enable a muscle to fine tune its efficiency for the wide range of forces, velocities, levels of endurance and levels of resistance to fatigue it is required to generate (Pette & Staron, 2000; Pette et al., 1999; Galler et al., 1994; Botinelli et al., 1994a,b; Danieli-Betto et al., 1986). This view, which does not distinguish between hybrid fibres from normal and transforming muscles, is based largely on data showing that some contractile characteristics of MHC hybrids lie between the contractile characteristics of the corresponding pure fibres. For example, in adult rat skeletal muscle, hybrid and pure fibre types have been found to display a continuum of values with respect to stretch-activation kinetics (Galler et al., 1994), maximum/unloaded shortening velocity (Botinelli et al., 1994a), myofibrillar ATPase and tension cost (the ratio between ATPase activity and isometric tension; Botinelli et al., 1994b).
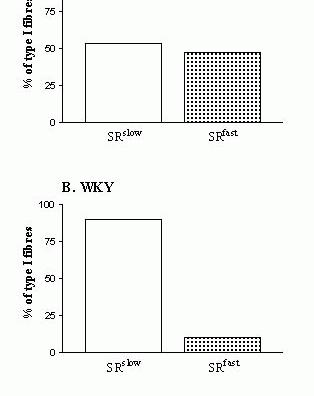
Figure 4. MHC-SR protein hybrids
(……….)
detected in soleus muscles from adult SHR (panel A) and WKY (panel B) male rats, based on caffeine thresholds for contraction. Methodological details and the data which were used to plot these graphs can be found in Bortolotto et al. (2000b).
Support for the idea that hybrid fibres enable a muscle to respond more effectively to functional demands comes not only from studies of contractile properties in fibres from normal and transforming muscles, but also from data on the fibre type composition of very specialised normal muscles. For example, Dammeijer et al. (2000) found that all fibres in rat stapedius, a small muscle believed to prevent the auditory receptors in the inner ear from injury by intense noise, co-expressed more than one MHC isoform. According to Dammeijer et al. (2000), this unusual fibre type composition enables the stapedius muscle to contract fast and fatigue slowly (thereby stiffening the middle ear bone chain) at acoustic stimulation stronger that 80 dB.
There are reasons to believe that ‘the contractile properties continuum’ paradigm tells only part of the story of the functional significance of hybrid fibres. Since it has been developed mainly on the basis of results obtained with MHC hybrids co-expressing two MHC isoforms only, the paradigm does not explain the role of hybrid fibres co-expressing three or four MHC isoforms (Talmadge, 2000) or displaying other types of polymorphism.
There is no doubt that far more work will have to be carried out in order to understand the functional significance of hybrid fibres in skeletal muscle. From a methodological point of view, this will mean in first instance applying the aforementioned strategies to more functional parameters, more muscles and more species. One can envisage, however, that this approach will not be sufficient and that other methods will have to be developed/refined later on. In this context, a study by Acakpo et al. (1997) in which mice carrying null mutations in members of the MHC gene family were used to examine the functional role of MHCIIb and MHC IId in mouse EDL and diaphragm may be regarded as a trendsetter.
Molecular mechanisms underlying the hybrid fibre phenomenon
Related to the functional significance of hybrid fibres is the issue of their origin. Some obvious questions that can be asked in connection with this issue are: (i) what are the initiating signal(s) and cellular pathway(s) involved in the appearance of hybrid fibres in transforming muscles? (ii) are the cellular events associated with muscle transformation muscle-specific or transforming factor/condition-specific? (iii) when and how do hybrids appear in normal muscles? (iv) is muscle protein polymorphism related to the presence of multiple nuclei in the muscle cell? (v) why do hybrids from transforming muscles have a more complex pattern of MHC isoform co-expression than hybrids from normal muscles? (vi) is the muscle protein complement expressed in a hybrid fibre predetermined or does it result from various inductive influences exerted on a naive cell? Once again, it is important to stress that our current understanding of the origin of hybrid fibres (particularly of those present in normal muscles) is very limited.
According to currently available data, most proteins involved in the E-C-R cycle are encoded by unique genes and their isoforms are generated by alternative splicing of the primary RNA transcript through a process mediated by muscle-specific factors. The most frequently cited examples of muscle protein isoforms produced by alternative splicing are the two fast-twitch isoforms of myosin light chains, MLC1f and MLC3 (Wade & Kedes, 1989).
Some muscle proteins (such as MHCs) are encoded, however, by multiple genes (referred to as a ‘multi-gene family’or ‘isogenes’) and their isoforms results from the differential expression of the gene-family members. It is interesting to note that isogenes can be located on the same chromosome (in tandem or as a cluster), but also on different chromosomes, and that their expression is regulated by muscle-specific cis-acting sequences (such as the E-box elements) and trans-acting factors (Talmadge 2000; Weiss & Leinwand, 1996).
Based on this insight into the molecular mechanisms underlying muscle protein polymorphism, it is reasonable to suggest that, in transforming muscles, the hybrid fibre phenotype is the product of a series of coordinated or independent transcriptional events initiated by transforming factors/conditions. In a recent review, Talmadge (2000) raised also the possibility that MHC hybrid fibres observed in skeletal muscle after alterations in electrical activity result from the differential responsiveness of individual myonuclei in a muscle fibre to regulators of MHC isoform gene expression. The idea of nuclei of transforming fibres not working in synchrony, which has been canvassed earlier by Staron & Pette (1987), provides an interesting perspective when inquiring into the origin of hybrid fibres in muscles in transition.
It has been suggested that the molecular heterogeneity of hybrid fibres can derive not only from pre-translational , but also from translational and even post-translational events. In the former case, the resulting hybrid fibres may contain one isoform expressed as a protein, and another expressed as a matched or mismatched mRNA species (Andersen et al., 1999; Barton & Buckingham, 1985). In the latter case, the presence of certain isoforms in a fibre may be the result of relatively slow rates of degradation. Thus, Staron & Pette (1993) argued that the MHCIIb isoform detected in hybrid fibres from rat fast-twitch muscles undergoing fast-to-slow transition is not a newly expressed protein, but a transient species with a half life of about 14.7 days. If the rates of degradation of muscle proteins are slow and if they are isoform-specific it is quite clear that after a sudden change in certain conditions there would be an isoform-specific lag between the time when the synthesis of a protein isoform stops and the time when the protein disappears completely from the cell. In this case, however, one would have to wonder about the functional status of the isoform that is in the process of being replaced by a newly synthesized one.
Experimental value of hybrid fibres.
In this review it has been argued that identifying and characterising hybrid fibres is not an easy task. Indeed, each of the methods used so far for this purpose has been found to be fraught with technical difficulties and, under certain conditions, to be of limited effectiveness. So, why study hybrid fibres?
The reasons for studying hybrid fibres become clearer if one considers a small sample of research questions that have already benefited or are likely to benefit in the future from studies of hybrid skeletal muscle fibres (Table IV). These questions, whose scope may at times overlap, belong loosely to two major fields of inquiry: one concerned with the relationship between the structure of muscle proteins and their specific roles in events of the E-C-R cycle (1a-11a), the other, of more general interest, concerned with mechanisms of regulation of gene expression in mammalian cells (1b –5b). Let us consider, for example, the paradigm of MHC gene-switching pathway in mammalian skeletal muscle. This is a paradigm that has emerged as a result of the discovery of hybrid fibres and is continuously modified to reflect the data generated by ongoing research on the diversity and plasticity of skeletal muscle fibres and on muscle protein polymorphism. Thus, shortly after the first reports of MHC isoform co-expression in single fibres, Danieli-Betto et al. (1986) hypothesised that during transition from slow to fast phenotype, MHC genes in adult rat skeletal muscle are activated in the sequence I → IIa → IIb. As a result of 14 years of intense investigations on muscles in transition, the paradigm has been modified to include the newly discovered fast-twitch MHCIId/x isoform (I → IIa → IId → IIb; Talmadge, 2000) and to reflect the reversible nature of fibre transition (MHCI ↔ MHC IIa ↔ MHC IId ↔ MHC IIb; Pette & Staron, 2000). As emphasized by Oishi et al. (1998), learning from hybrid fibres the MHC composition in a fibre, at the start of the transforming process, is essential when defining the specific steps in the MHC activation sequence.
| Research question |
| Questions related to the relationship between the structure and function of proteins involved in the E-C-R cycle. |
| 1a. What is the distribution of hybrid fibres in a given muscle and how does it relate to the overall muscle function? |
| 2a. Do motor units contain hybrid fibres? |
| 3a. When several MHC isoforms are present in the same muscle fibre, are they expressed simultaneously or is there a programmed gene switching process |
| 4a. Do MLCs play a role in mATPase activity? |
| 5a. Is the pattern of muscle protein co-expression in a hybrid fibre constant along the length of the fibre? |
| 6a. What are the relative rates of synthesis/degradation of various muscle protein isoforms in skeletal muscle? |
| 7a. Are MHC and MLC isoforms independently regulated? |
| 8a. What is the origin of hybrid fibres in normal muscles? |
| 9a. Are there any molecular differences between hybrid fibres from transforming muscles and normal muscles? |
| 10a. Are interactions between mismatched isoforms of myosin subunits (eg. MHCI and MLC1f) different from those between matched isoforms (eg. MHCI and MLC1s)? |
| 11a Can the proportion or type of hybrid fibres act as an indicator of skeletal muscle pathology? |
|
Questions related to the mechanisms of regulation of gene expression in complex eukaryotic cells |
| 1b. What is the subcellular distribution of isoform-specific mRNA species? |
| 2b. Are events involved in the synthesis of various muscle protein coordinated? |
| 3b. Are events involved in the degradation of various muscle protein coordinated? |
| 4b. What are the superior elements controlling the coordinated expression of genes regulated by gene switching and by alternative splicing |
| 5b. How do different nuclei work in a multinucleated cell? |
There is little doubt that, in terms of attention received from muscle researchers, the ‘hybrid’ fibre (regardless of its type) has reached the big time. This is not surprising, for as Pette et al. (1999) stated recently, these fibres ‘offer unique opportunities for relating molecular patterns of protein expression to functional properties and for elucidating mechanisms controlling gene expression in muscle’.
Conclusions
The major points made in this review can be summarized as follows:
•
Hybrid fibres are present in both normal and transforming skeletal muscle, but their proportion and
molecular complexity is higher in the latter. The frequency of their occurrence in various muscles and species suggests that hybrid fibres are not a rare phenomenon.
•
To date, the phrase ‘hybrid fibres’ has been used to describe only fibres co-expressing several
MHC isoforms. MHC hybrids represent, however, only one of the many types of hybrid fibres that exist in vertebrate skeletal muscles. Therefore, in order to facilitate progress in the area concerned with muscle fibre diversity and plasticity, a new set of terms is required. This new terminology should be able to include all the hybrid fibre types discovered so far, as well as those that are likely to be discovered in the future.
•
To detect and characterize hybrid muscle fibres one needs to combine creatively existing
microanalytical, microphysiological and microhistochemical methods and to develop new methods for single fibre analysis.
•
Regardless of their type, hybrid fibres have the potential to become valuable tools for the pursuit of
knowledge related to events of the E-C-R cycle and to the regulation of gene expression in multinucleated cells.
Acknowledgements
The support provided by NH&MRC, ARC and Vera Ramaciotti Foundation to the Muscle Cell Biochemistry Laboratory at Victoria Institute of Technology, for work on hybrid fibres, is gratefully acknowledged.
References
Acakpo-Satchivi, L.J.R., Edelmann, W., Sartorius, C., Lu, B.D., Wahr, P.A., Watkins, S.C., Metzger, J., Leinwand, L & Kucherlapati, R. (1997) Growth and muscle defects in mice lacking adult myosin heavy chain genes. Journal of Cell Biology, 139, 1219-1229.
Aigner, S., Golsch, B., Hämäläinen, N., Staron, R.S., Uber, A., Wehrle, U. & Pette, D. (1993) Fast myosin heavy chain diversity in skeletal muscles of the rabit: heavy chain IId, not IIb predominates. European Journal of Biochemistry, 211, 367-372.
Andersen, J.L., Gruschi-Knudsen, T., Sandri, C., Larsson, L. & Schiaffino, S. (1999) Bed rest increases the amount of mismatched fibres in human skeletal muscles. Journal of Applied Physiology, 86, 455-460.
Armstrong, R.B. & Phelps, R.O. (1984) Muscle fibre type composition of the rat hindlimb. American Journal of Anatomy, 171, 259-272.
Barton, P.J. & Buckingham, M.E. (1985) The myosin light chain proteins and their genes. Biochemical Journal, 231, 249-261.
Bortolotto, S.K., Cellini, M., Stephenson, D.G.& Stephenson, G.M.M. (2000a) MHC isoform composition and Ca2+- or Sr2+-activation properties of rat skeletal muscle fibres. American Journal of Physiology, 279, 1564-1577
Bortolotto, S.K., Stephenson, D.G.& Stephenson, G.M.M. (2000b) Caffeine thresholds for contraction in electrophoretically typed, mechanically skinned muscle fibres from SHR and WKY rat. Pflügers Archiv (in print).
Bortolotto, S.K., Stephenson, D.G.& Stephenson, G.M.M. (1999) Fibre type populations and Ca2+- activation properties of single fibres in soleus muscles from SHR and WKY rats. American Journal of Physiology, 276, C628-C637.
Bottinelli, R., Betto, R., Schiaffino, S & Reggiani, C. (1994a) Maximum shortening velocity and coexistence of
myosin heavy chain isoforms in single skinned fast fibres of rat skeletal muscle. Journal of Muscle Research and Cell Motility, 15, 413-419.
Bottinelli, R., Canepari, M., Reggiani, C. & Stienen, G.J.M. (1994b) Myofibrillar ATP ase activity during isometric contraction and isomyosin composition in rat single skinned muscle fibres. Journal of Physiology, 481.3, 663-675.
Csernoch, L. (1999) Regulation of the ryanodine receptor calcium release channel of the sarcoplasmic reticulum in skeletal muscle. Acta Physiologica Hungarica, 86, 77-97.
Dammeijer, P.F.M., Van Mameren, H., Van Dijk, P., Moorman, A.F.M., Habets, P., Manni, J.J. & Drukker, J. (2000) Stapedius muscle fibre composition in the rat. Hearing Research, 141, 169-179.
Danieli-Betto, D., Betto, R. & Midrio, M. (1990) Calcium sensitivity and myofibrillar protein isoforms of rat skinned skeletal muscle fibres. Pflügers Archiv, 417, 303-308.
Danieli-Betto, D., Zerbato, E. & Betto, R. (1986) Type I, 2A, and 2B myosin heavy chain electrophoretic analysis of rat muscle fibres. Biochemical and Biophysical Research Communications, 138, 981-987.
Dix, D.J. & Eisenberg, B.R. (1988) In situ hybridization and immunohistochemistry in serial sections of rabbit skeletal muscle to detect myosin expression. Journal of Histochemistry and Cytochemistry, 36, 1519-1526.
Fink, R.H., Stephenson, D.G. and Williams, D.A. (1986) Calcium and strontium activation of single skinned muscle fibres of normal and dysstrophic mice. Journal of Physiology, 373, 513-525.
Galler, S., Schmitt, T.L. & Pette, D. (1994) Stretch activation, unloaded shortening velocity, and myosin heavy chain isoforms of rat skeletal muscle fibres. Journal of Physiology, 478, 513-521.
Geiger, C.G., Cody, M.J. & Sieck, G.C. (1999) Force-calcium relationship depends on myosin and troponin isoforms in rat diaphragm muscle fibres. Journal of Applied Physiology, 87, 1894-1900.
Grossman, E.J., Roy, R.R., Talmadge, R.J., Zhong, Hui. & Edgerton, V.R. (1998) Effects of inactivity on myosin heavy chain composition and size of rat soleus fibres. Muscle & Nerve, 21, 375-389.
Hämäläinen, N. & Pette, D. (1995) Patterns of myosin isoforms in mammalian skeletal muscle fibres. Microscopy Research and Technique, 30, 381-389.
Kischel, P., Bastide, B., Stevens, L. & Mounier, Y. (2001) Expression and functional behaviour of troponin C in soleus muscle fibres of rat after hindlimb unloading. American Journal of Applied Physiology, (In press).
La Framboise, W.A., Daood, M.J., Guthrie, R.D., Schiaffino, S., Moretti, P., Brozanski, B., Ontell, M.P., Butler-Browne, G.S., Whalen, R.G & Ontell, M. (1991) Emergence of the mature myosin phenotype in the rat diaphragm muscle. Developmental Biology, 144, 1-15.
Loukianov, E., Ji, Y., Baker, D.L., Reed, T., Babu, J., Loukianova, T., Greene, A., Shull, G & Periasamy, M. (1998) Sarco(endo)plasmic reticulum Ca2+ ATPase isoforms and their role in muscle physiology and pathology. Annals of the New York Academy of Sciences, 853, 251-259.
Lutz, G.J. & Lieber, R.L. (2000) Myosin isoforms in anuran skeletal muscle: their influence on contractile properties and in vivo muscle function. Microscopy Research and Technique, 50, 443-457.
Mizusawa, H., Takagi, A., Sugita, H. & Toyokura, Y. (1982) Coexistence of fast and slow types of myosin light chains in a single fiber of rat soleus muscle. Journal of Biochemistry (Tokyo), 91, 423-425.
Moss, R.L., Diffee, G.M. & Greaser, M.L. (1995) Contractile properties of skeletal muscle fibres in relation to myofibrillar protein isoforms. Reviews in Physiology, Biochemistry and Pharmacology, 126, 2-63.
Nguyen, L.T. & Stephenson, G.M.M. (1999) An electrophoretic study of myosin heavy chain expression in skeletal muscles of the toad Bufo marinus. Journal of Muscle Research and Cell Motility, 20, 687-95.
Oishi, Y., Ishihara, A., Yamamoto, H. & Miyamoto, E. (1998) Hindlimb suspension induces the expression of multiple myosin heavy chain isoforms in single fibres of the rat soleus muscle. Acta Physiologica Scandinavica, 162, 127-134.
Pette, D., Peuker, H. & Staron, R.S. (1999) The impact of biochemical methods for single muscle fibre analysis. Acta Physiologica Scandinavica, 166, 261-277.
Pette, D. & Staron, R.S. (2000) Myosin isoforms, muscle fibre types and transitions. Microscopy Research and
Technique, 50, 500-509.
Pette, D. & Staron, R.S. (1997) Mammalian skeletal muscle fibre type transitions. International Review of
Cytology, 170, 143-223.
Pette, D. & Staron, R.S. (1990) Cellular and molecular diversities of mammalian skeletal muscle fibres. Reviews
of Physiology, Biochemistry and Pharmacology, 116, 2-76.
Peuker, H., Conjard, A., Putman, C.T. & Pette, D. (1999) Journal of Muscle Research and Cell Motility, 20, 147-154.
Pierobon-Bormioli, S., Sartore, S., Libera, L.D., Vitadello, M. & Schiaffino, S. (1981)"Fast" isomyosins and fiber types in mammalian skeletal muscle. Journal of Histochemistry and Cytochemistry, 29, 1179-1188.
Rivero, J.L., Talmadge, R.J. & Edgerton, V.R. (1998) Fibre size and metabolic properties of myosin heavy chain-based fibre types in rat skeletal muscle. Journal of Muscle Research and Cell Motility,19, 733-742.
Roy, R.R., Kim, J.A., Grossman, E.J., Bekmezian, A., Talmadge, R.J., Zhong, H. & Edgerton, V.R. (2000) Persistence of myosin heavy chain-based fiber types in innervated but silenced rat fast muscle. Muscle & Nerve, 23, 735-747.
Salviati, G., Betto, R.& Danieli-Betto, D. (1982) Polymorphism of myofibrillar proteins of rabbit skeletal
muscle fibres. Biochemical Journal, 207, 261-272.
Schiaffino, S. & Salviatti, G. (1998) Molecular diversity of myofibrillar proteins: isoforms analysis at the protein and mRNA level. Methods in Cell Biology, 52, 349-369
Schiaffino, S. & Reggiani, C. (1996) Molecular diversity of myofibrillar proteins: gene regulation and functional significance. Physiological Reviews, 76, 371-423.
Staron, R.C. & Pette, D. (1987) Nonuniform myosin expression along single fibres of chronically stimulated and contralateral rabbit tibialis anterior muscles. Pflügers Archiv, 409, 67-73.
Staron, R.C. & Pette, D. (1993) The continuum of pure and hybrid myosin heavy chain based fibre types in rat skeletal muscles. Histochemistry, 100, 149-153.
Stephenson, D.G., Lamb, G.D. & Stephenson, G.M.M. (1998) Events of the excitation-contraction-relaxation cycle in fast-and slow-twitch mammalian muscle fibres relevant to muscle fatigue. Acta Physiologica Scandinavica, 162, 229-245.
Swynghedauw, B. (1986) Developmental and functional adaptation of contractile proteins in cardiac and skeletal muscles. Physiological Reviews, 66, 710-771.
Talmadge, R.J. (2000) Myosin heavy chain expression following reduced neuromuscular activity: potential regulatory mechanisms. Muscle & Nerve, 23, 661-679.
Talmadge, R.J., Roy R.R. & Edgerton, V.R. (1999) Persistence of hybrid fibres in rat soleus after spinal cord transection. Anatomical Records, 255, 188-201.
Termin, A., Staron, R.S. & Pette, D. (1989) Changes in myosin heavy chain isoforms during chronic low-frequency stimulation of rat fast hindlimb muscles. European Journal of Biochemistry, 186, 749-754.
Van der Linden, G.C., Simonides, W.S., Muller, A., Van der Laarse, W.J., Vermeulen, j.L., Zuidvijk, M.J., moorman, A.F. & van Hardeveld, C. (1996) Fibre-specific regulation of Ca2+ - ATPase isoform expression by thyroid hormone in rat skeletal muscle. American Journal of Physiology, 271, C1908-C1919.
Wade, R. & Kedes, L. (1989) Developmental regulation of contractile protein genes. Annual Review of
Physiology, 51, 179-188.
Weiss, A. & Leinwand, L.A. (1996) The mammalian myosin heavy chain gene family. Annual. Review of
Cellular and Developmental Biology, 12, 417-39.
Wu, Y.Z., Baker, M.J., Crumley, R.L., Blanks, R.H. & Caiozzo, V.J. (1998) A new concept in laryngeal muscle: multiple myosin isoform typesin single muscle fibers of the lateral cricoarytenoid. Otolaryngology of the Head Neck Surgery, 118, 86-94.
Wu, Z.Y., Baker, M.J., Crumley, R.L. & Caiozzo, V.J. (2000a) Single-fiber myosin heavy chain isoform
composition of rodent laryngeal muscle. Modulation by thyroid hormone. Archives of Otolaryngological Head and neck Surgery, 126, 874-880.
Wu, Y.Z., Crumley, R.L. & Caiozzo, V.J. (2000b) Are hybrid fibers a common motif of canine laryngeal muscles? Archives of Otolaryngological Head and Neck Surgery, 126, 865-873.
Yu, F., Degens, H., Li, X & Larsson, L. (1998) Gender- and age-related differences in the regulatory influence of thyroid hormone on the contractility and myosin composition of single rat soleus muscle fibres. Pflügers Archiv, 437, 21-30.