Abnormal extracellular Ca2+ influx has been suggested to be involved in the process of muscle wasting in Duchenne muscular dystrophy. However, studies comparing the resting intracellular Ca2+ levels in normal and dystrophic muscle cells from patients with Duchenne muscular dystrophy and mdx mice have yielded contradictory findings (Gillis, 1996). Ca2+ indicators targeted to the inner sarcolemmal membrane have recently been reported to be more sensitive to sarcolemmal Ca2+ influx than standard cytosolic Ca2+ indicators such as fura-2 (Bruton et al., 1999). In this study, we measured the resting Ca2+ levels and Ca2+ transients in myotubes grown from mdx and normal mice using the near-membrane Ca2+ indicator FFP-18.
Skeletal muscle satellite cells were isolated from the hind limbs of neonatal normal and mdx mice that had been killed by decapitation. Myotubes were grown on glass coverslips coated with collagen. The myotubes were loaded with the Ca2+ indicator by exposure to FFP-18-AM (3 μM) and 0.0125% Pluronic F-127 for 45 min at room temperature (22-23°C). Ca2+ measurements were made with a Cairn spectrophotometer attached to a Nikon inverted microscope equipped for epifluorescence. The myotubes were stimulated by electrical field stimulation (EFS) via two small platinum wires (single 0.2 ms pulse).

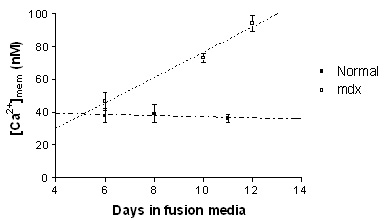
Resting near membrane [Ca2+] ([Ca2+]mem) levels increased significantly during development in the mdx myotubes, (slope; 8.19 ± 1.47, p<0.0001). However, no change in [Ca2+]mem was found in normal myotubes during development (slope; -0.40 ± 1.14, p=0.73). From the fitted lines, the [Ca2+]mem in 12 days old mdx and normal myotubes was estimated at 93 and 36 nM respectively (Figure). Increasing the driving force for Ca2+ influx by raising extracellular Ca2+ to 18 mM, increased the steady state [Ca2+]mem by 156.1 ± 14.2 % (to ~ 208 nM) (n=14) in mdx myotubes, while in normal myotubes, the [Ca2+]mem increased by only 28.8 ± 7.6 % (to ~ 49 nM) (n=6), (p=0.007, unpaired Student's t-test). The half-relaxation time of EFS-induced Ca2+ transients was significantly increased in mdx (314.5 ± 36.9 ms, n=8) compared to normal myotubes (163.3 ± 28.4 ms, n=6) (p=0.01, unpaired t-test), which is consistent with previous studies using standard Ca2+ indicators.
The results of this study further support the hypothesis that increased Ca2+ influx results in raised intracellular levels in dystrophin-deficent skeletal muscle cells. The use of FFP-18 to measure steady state cytosolic Ca2+ in normal and mdx myotubes in the presence of raised extracellular Ca2+ could provide a more reliable method for detecting the altered Ca2+ homeostasis in dystrophic muscle cells.
Bruton J.D., Katz A. & Westerblad H. (1999) Proceedings of the National Academy of Sciences USA, 96, 3281-3286.
Gillis J.M. (1996) Acta Physiologica Scandinavica, 156, 397-406.