The maintenance of adequate levels of the cell antioxidant glutathione (GSH) is essential for defence against oxidative disruption of membranes, proteins and DNA. These vital cell constituents are protected by the preferential oxidisation of GSH to glutathione disulfide (GSSG) which is rapidly reduced back to GSH by NAPDH and glutathione reductase. Recycling of GSSG to GSH allows for slow replacement of total glutathione (GSH & GSSG; TG) with a turnover time of six days in red blood cells (RBCs). However, oxidative stress can cause accumulation of GSSG and its export from the cell. Under such circumstances, the rate of TG synthesis is thought to be increased by reduced product inhibition by GSH on the rate limiting enzyme γ-glutamyl-cysteine synthase.
GSH synthesis has been studied using lysates and purified enzymes, but little is known of the control of GSH production within whole RBC. Although sensitive and specific spectrophotometric methods have long been available for measuring TG (Tietze, 1969), its synthesis in RBC is too slow to measure in this way particularly against the high concentrations (2.3 mmol(l RBC)-1) normally present.

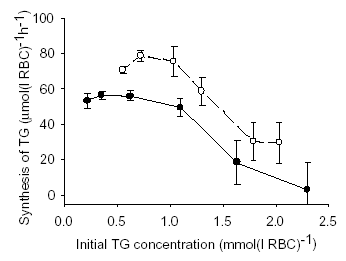
GSH is also active in glutathione S-transferase catalysed conjugation with hydrophobic, electrophilic compounds including toxins and drugs. Glutathione S-conjugates are actively exported from RBCs. One such S-transferase substrate 1-chloro-2,4-dinitrobenzene (CDNB) has been used extensively for depleting cell TG content for experimental purposes. For RBCs suspended at a 10% haematocrit in buffer containing 2 mM dithiothreitol, I found that incubation for 40 minutes at 37°C with CDNB concentrations from 0.08 to 0.40 mM reduced the TG levels by 30 to 90%, respectively. When the TG depleted RBCs were washed free of the water soluble glutathione-CDNB conjugate and resuspended in a HEPES buffered solution containing 5 mM N-acetylcysteine and glucose, the TG levels in the RBC, measured by a modified version of Tietze’s assay, increased in a linear fashion for several hours. The rate of TG production was dependent on the degree of depletion of TG with a maximum rate of synthesis of 72.6 ± 15.0 μmol(l RBC)-1h-1 (mean ± SD) which is a five-fold increase on rates previously measured in RBCs with normal levels of GSH (Griffith, 1981). The results of 2 of 5 such experiments are presented in the Figure. The error bars are the SE of the rates of synthesis returned by linear regression. Buthionine sulfoximine a potent inhibitor of γ-glutamyl-cysteine synthase was shown to inhibit the rate of TG increase in RBC by 55% confirming that the accumulation of TG observed was due to synthesis. The controlling factors of TG synthesis within whole RBCs will be determined by measuring TG production under various experimental conditions. Once identified, manipulation of controlling factors may be used to prevent depletion or stimulate production of GSH in several disease states characterised by reduced cell TG.
Griffith, O.W. (1981) Journal of Biological Chemistry 256, 4900-4.
Tietze, F. (1969) Analytical Biochemistry 27, 502-22.