The epithelial sodium channel (ENaC) plays a critical role in regulating body sodium and fluid homeostasis. Activity of ENaC is regulated by hormones such as aldosterone and insulin. Aldosterone increases ENaC activity within 30 min and this early effect of the hormone is non-genomic. Although the mechanisms that underlie the early effect of aldosterone on ENaC activity are still unknown, it is believed that this effect may be mediated by increasing insertion of ENaC from a cytosolic pool into the cell membrane. To investigate this hypothesis, we used a retroviral expression system and Ussing chamber technique to study regulation of ENaC activity in the mouse collecting duct (M1) cell line. To investigate insertion of ENaC, a glycine codon in the amiloride-sensitive region of the γENaC gene was mutated to cysteine (G542C). A retrovirus expressing this gene was constructed and used to stably transfect M1 cells. The M1 cells that were transfected with G542CγENaC were selected using neomycin resistance and then cultured on Millicell-CM for 14-16 days until the transmembrane resistance was fully developed. The Ussing chamber method was used to measure equivalent short circuit current (Iesc) across the monolayer. The G542C mutation makes ENaC sensitive to [2- (trimethylammonium) ethyl] methanethiosulfonate bromide (MTSET). MTSET forms a disulfide bond with cysteine and inhibits ENaC activity irreversibly. Recovery rate of the MTSET-sensitive current was then used to measure the rate of insertion of active ENaC into the cell membrane.
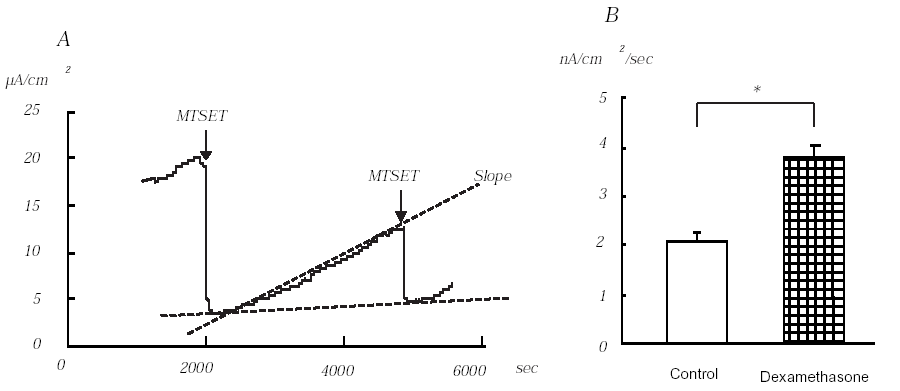
One hour before each experiment, 100 nM dexamethasone was added to the culture medium. Application of MTSET to the apical side of the monolayers decreased Iesc from 13.0 ± 0.8 to 2.6 ± 0.1μA/cm2 (n=10). Dexamethasone significantly increased baseline MTSET-sensitive Iesc from 10.3 ± 0.8 μA/cm2 (n=10) to 19.1 ± 1.2 μA/cm2 (n=9, P<0.01). After the maximum inhibitory effect of MTSET was observed, the chamber was perfused with HEPES solution for 45 minutes and the slope of MTSET-sensitive Iesc was measured (A in Figure). The slope measured in the dexamethasone treated group (3.8 ± 0.2 nA/cm2/s) was significantly higher than that of the control group (2.1 ± 0.1 nA/cm2/s) (B in Figure, P<0.001). The second MTSET sensitive Iesc recovered to 50% of original MTSET sensitive Iesc in both control and dexamethasone groups. Our study, therefore, clearly indicates that the early effect of dexamethasone on Na+ transport in M1 cells is mediated by increasing insertion of ENaC into the cell membrane.
