Very large changes in plasma progesterone and oestradiol are observed during late pregnancy, in Australia's greyheaded flying-fox, Pteropus poliocephalus, but changes associated with ovulation are enigmatic. Attempts to identify the time of ovulation by monitoring peripheral hormones have been so unsuccessful that a question has arisen as to the nature of feedback between the ovary and the hypothalamus-pituitary axis in these animals. P. poliocephalus mate during April-May and deliver a single young in October-November. In captivity they can live for 20-30 years but wild populations are listed as ‘threatened’ under the Environment Protection and Biodiversity Conservation Act. Understanding their reproductive physiology is essential for recovery of the species. Important stages of reproduction, including ovulation, are regulated by luteinising hormone (LH) but plasma LH has not previously been measured in the Order Megachiroptera. Recent development of an assay for flying-fox LH (O'Brien et al., in press) has made it possible to test for the effects of reproductive stage (copulation, pregnancy etc.) and ovarian feedback on mean plasma LH levels in female flying-foxes through an entire annual cycle of reproduction.
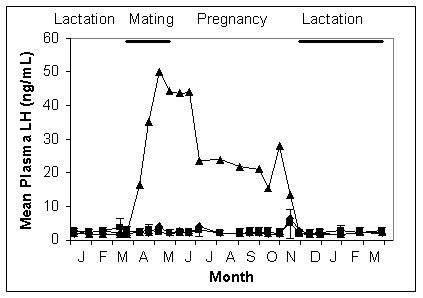
Methods. Blood samples were collected by venipuncture (O'Brien et al., 1996), at intervals of 1 to 4 weeks. LH was measured by 125I-RIA using monoclonal antibody 518B7, and ovine LH (oLH-G3-330-Br) as radioligand and standard (O'Brien et al., in press). Animals were housed in a single-sex group (segregated ◆ n=4) or in a large mixed-sex group (breeding ■ sub-set n=5); each group had a long-term ovariectomised female with them (ovariectomised ▲ data are average of 2).

Results shown in the Figure clearly demonstrate three phases of LH secretion during the year in the absence of ovarian steroid feedback (ovariectomised ▲): very high levels Apr-May; elevated levels Jun-Oct; baseline levels Nov-Mar. Mean plasma LH remained at baseline all year in intact animals (◆, ■: Figure shows means and SD).
Discussion. The pituitary appears to be programmed to stimulate the ovaries from mid-autumn (April) until mid-spring (October) (see the Figure). Pituitary content of LH is high during April-May (O'Brien et al., in press). However strong negative feedback from the ovaries reduced the frequency and/or amplitude of LH pulses so dramatically that no elevation of mean plasma levels was observed in intact animals. There was no indication of the timing of ovulation. During pregnancy, placental steroids probably also contributed to the feedback.
The phase of the central program revealed during June-October probably provides a safety net in the event of early pregnancy loss and if so, would explain the occasional late births that are recorded. It may also provide the physiological substrate for the observed geographic variation in breeding times of a congeneric species, P. alecto (Vardon & Tidemann 1998).
The November-March phase reveals a centrally programmed anoestrus not previously known. This may explain why variations in the timecourse of lactation do not influence timing of the subsequent pregnancy (unpub. obs.).
O'Brien, G.M., Curlewis, J.D. & Martin, L. (1996) General & Comparative Endocrinology 104, 304-311.
O'Brien, G.M., McFarlane, J.R. & Kearney, P.J. (in press) Reproduction, Fertility and Development.
Vardon, M.J. & Tidemann, C.R.. (1998) Australian Journal of Zoology, 46, 329-344.