1. Activated Ca2+-sensing receptors (CaRs) play key roles in the regulation of whole body calcium metabolism by inhibiting the secretion of the key calcitropic hormone, PTH and promoting urinary calcium excretion.
2. We have now examined the effects of intravenous administration of novel calcium receptor activators on renal function in anaesthetized female Wistar rats.
3. The type-II calcimimetic, NPS R467 and the CaR-active amino acids, L-Phe and L-Ala, which act at distinct binding sites on the receptor all activated urinary flow rate, calcium and osmolar excretion and suppressed urinary osmolality.
4. The effects of L-Phe and NPS R-467 on urine flow rate and calcium excretion were stereoselective consistent with the idea that these effects were mediated by calcium-sensing receptors.
5. However, D-Phe also suppressed urinary osmolality and promoted osmolar excretion possibly by exceeding the transport maximum in the proximal tubule.
6. The data indicate that novel activators of calcium-sensing receptors, including L-amino acids at physiologically relevant serum concentrations, play a significant role in the regulation of urinary calcium and water excretion.
The calcium-sensing receptor (CaR) is a member of group C of the G-protein coupled receptor super-family. These receptors play multiple roles in calcium homeostasis including key roles in mediating the feedback regulation of parathyroid hormone secretion and urinary calcium excretion. Inactivating mutations of the CaR underlie several human pathological states including the relatively benign condition familial hypocalciuric hypercalcemia and its more severe but much rarer homozygous form, neonatal severe hyperparathyroidism which requires parathyroidectomy within the first few weeks of life (review:1). The widespread distribution of these receptors, together with their resistance to desensitization, points to much wider roles in mammalian physiology (review:2).
Recently, two new classes of calcium-sensing receptor (CaR) activators have been identified. The type-II calcimimetics (e.g., NPS R-467 and R-568) were developed from a lead phenylalkylamine compound identified in a large-scale drug screen3. Type-II calcimimetics sensitize the CaR to calcium ions by binding to a site in the receptor’s transmembrane region4. More recently, several sub-classes of L-amino acids (including aromatic, polar, and aliphatic amino acids) have been shown to act as allosteric activators of the CaR. Furthermore, physiologically relevant fluctuations in the concentration of physiological amino acid mixtures can modulate receptor activity5. The amino acid binding site is likely to lie in the conserved N-terminal, Venus FlyTrap domain6.
In the kidney, the CaR is expressed in multiple sites. These include the apical membrane of the proximal tubule, the basolateral membrane of the cortical thick ascending limb (CTAL) and the apical membrane of the medullary collecting ducts (review:7). Thus, fluctuations in the serum levels of Ca2+ and amino acids might be expected to modulate CaR activity in the CTAL and fluctuations in the tubular fluid levels of Ca2+ and amino acids might be expected to modulate CaR activity in the proximal tubule and collecting ducts. Consistent with a role for the CaR in the regulation of urinary phosphate excretion, dietary phosphate loading has been shown to suppress the expression of the CaR in the apical brush border membrane of the proximal tubule8. Furthermore, the CaR agonist gadolinium (Gd3+) and the type-II calcimimetic NPS R-467 reversed PTH suppression of phosphate reabsorption in cultured proximal tubule cells9. On the other hand, expression of the CaR in the CTAL has been linked to the control of urinary calcium excretion and expression of the CaR in the collecting tubule has been linked to the control of urinary water excretion and osmolality. In particular, CaR activation suppresses vasopressin-induced water reabsorption, facilitating the excretion of solutes such as calcium, phosphate and oxalate that might otherwise contribute to the formation of renal calculi10.

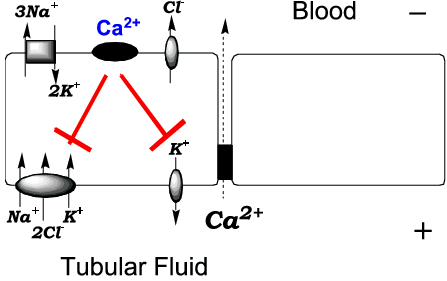
Figure 1. Schematic diagram of a thick ascending limb cell. The diagram shows the inhibitory effect of calcium-sensing receptor activation on apical Na+/K+/2Cl- co-transport and K+ recycling. The impact of CaR activation is believed to be a reduction in the lumen-positive potential difference that drives Ca2+ reabsorption.
The patterns of expression described above imply roles for CaR activators in the regulation of multiple renal functions including proximal tubular transport, calcium excretion and urinary concentration. For example, CaR-active amino acids (e.g., L-Phe and L-Ala) and type-II calcimimetics are predicted to promote calcium excretion (Fig. 1) and raise urine flow and suppress urinary osmolality (Fig. 2). We have examined the impact of intravenously administered L-amino acids or the type-II calcimimetic, NPS R-467 on renal calcium and water excretion in rats and report herein our preliminary findings. The data provide support for the hypotheses that CaR activators including L-amino acids promote urinary calcium and water excretion.
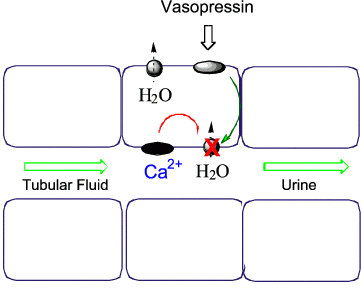
Figure 2. Schematic diagram of an epithelial cell from the collecting tubules. The diagram shows the inhibitory effect of CaR activation on vasopressin stimulated water reabsorption via aquaporin-2. In this way, CaR activators that have entered the renal filtrate and have not been reabsorbed in the proximal nephron may promote urinary water excretion.
NPS R-467 and its 100-fold less potent isomer S-467 were the generous gifts of Dr Edward Nemeth (NPS Pharmaceuticals, Toronto, Canada). Animal experiments on a total of approximately forty rats were performed with approval from the University of Sydney Animal Ethics Committee. Female Wistar rats (200-300 g) were anaesthetized with halothane (2% in oxygen; 0.8 mL/min) then catheterized. Both jugular veins were cannulated and the animals were infused at a constant rate (4 or 5 mL per h) with an isotonic physiological saline solution of the following composition: 140 mM NaCl, 4.0 mM KCl, 15 mM NaHCO3, 2.5 mM CaCl2, 1 mM MgCl2. After a 60 min equilibration period, continuous infusions of D and/or L-amino acids were commenced, continuing for 60 min prior to return to the control solution. Blood samples (0.25 mL) were collected at regular intervals for analysis of serum creatinine, osmolality, total calcium and various amino acids. Urine samples were collected at 15 min intervals to assess flow rate, osmolality and the concentrations of creatinine, calcium and amino acids. Osmolality was determined by vapour pressure osmometry. Creatinine was determined by an adaptation of the alkaline picrate method11 using a Wallac Victor2 multi-well plate reader and serum and urine total calcium concentrations were determined using an autoanalyzer (Roche/Hitachi 912). Amino acid levels in serum and urine were determined by HPLC separation and fluorimetric detection of O-phthaldialdehyde-conjugates12. In some experiments, bolus injections were administered to test for acute effects of R-467, S-467 and amino acids including L-Phe and L-Ala. The data are routinely expressed as means ± SEM (number of experiments).
The type-II calcimimetic R-467 administered as a bolus intravenous injection of 2 µmol stereoselectively enhanced urinary calcium excretion (by 3-4 fold; Fig. 3A) and also promoted urinary flow rate (Fig. 3B). In addition, R-467 stereoselectively suppressed urinary osmolality from a baseline level of 946 ± 70 mosm/kg to 723 ± 80 mosm/kg (n = 4; p = 0.01) after 15 min consistent with an inhibitory action of the CaR on vasopressin-induced water reabsorption in the collecting ducts. Although the osmolality dropped, the osmolar excretion rate increased following exposure to R-467. The baseline osmolar excretion rate was 12.5 ± 2.4 µosmol/min and this increased to 34.6 ± 0.8 µosmol/min following R-467 (n = 3; p = 0.01). R-467 also lowered serum total calcium levels (not shown) as previously reported for the related calcimimetic R-56813. The 100-fold less potent isomer, S-467 was much less effective than R-467 on all of the parameters tested.
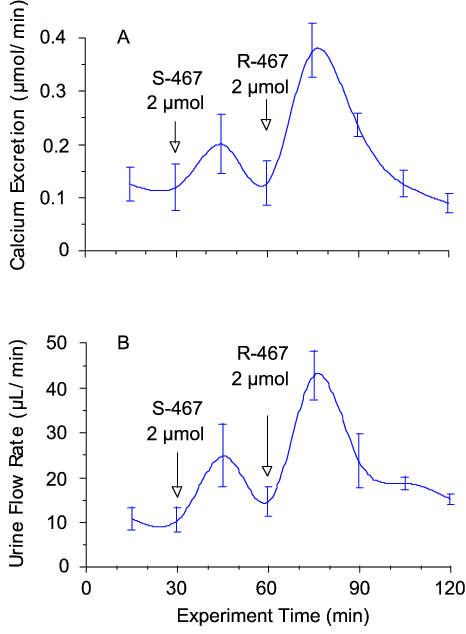
Figure 3. Effects of NPS R-467 on urinary calcium excretion and flow rate. Female Wistar rats were anaesthetised and infused intravenously with physiological saline at a rate of 4 mL/h via a jugular vein cannula. The CaR activator, R-467 and its stereoisomer S-467 were delivered intravenously as bolus injections (0.5 mL) in physiological saline. The data are means ± SEM (n=4).
Infusions of the CaR-active L-amino acid, L-Phe sufficient to raise the serum level from 0.05 mM to about 2 mM (determined by HPLC), also elevated urinary calcium excretion (by about 2-fold; Fig. 4A) and urinary flow rate (Fig. 4B). Both effects were L/D selective (Fig. 4). In addition, both D-Phe and L-Phe reversibly suppressed urinary osmolality. In the case of L-Phe, urinary osmolality was maximally suppressed from 767 ± 20 mosm/kg to 599 ± 17 mosm/kg (n = 3) after 60 min. The reason for the apparent lack of L/D selectivity of this effect is not clear. However, it may have arisen from higher local D-amino acid concentrations in the tubular fluid as a result of the selectivity of proximal tubular amino acid transporters for L-amino acids. In addition, the osmolar excretion rate was significantly increased following exposure to L-Phe and D-Phe. The baseline osmolar excretion rate was 13.2 ± 3.6 µosmol/min and this increased to 33.4 ± 4.2 µosmol/min following D-Phe (n = 3; p < 0.01) and 36.7 ± 5.3 µosmol/min following L-Phe (n = 3; p < 0.01). Bolus injections of L-Phe and L-Ala also acutely elevated urinary calcium excretion and flow rate and lowered urinary osmolality (not shown).
Taken together the data are consistent with the idea that novel activators of the CaR including L-amino acids and type-II calcimimetics such as R-467 mimic the effects of elevated plasma Ca2+ concentration on urinary calcium excretion, flow rate and osmolality. Increased serum and urinary amino acid concentrations associated, for example, with elevated dietary protein intake appear to act as physiological signals for enhanced solute and water excretion. CaR activators from distinct structural groups and targeting distinct binding sites on the receptor have similar effects on renal calcium and water excretion consistent with the proposed sites of CaR action in the thick ascending limb and collecting tubules.
The author’s work is supported by the NHMRC of Australia.
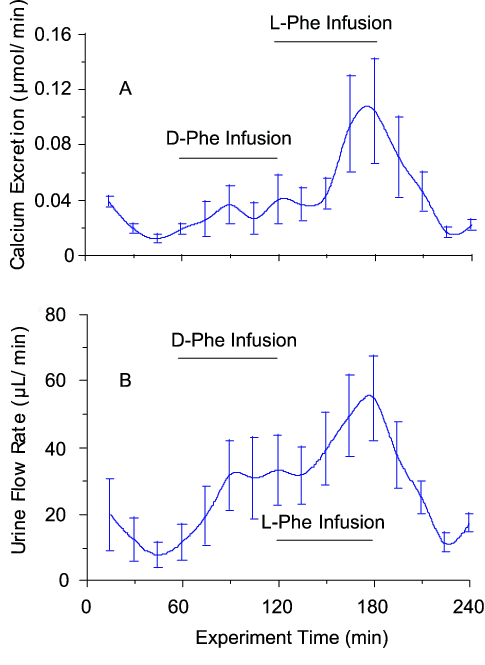
Figure 4. Effects of L-Phe and D-Phe infusions on urinary calcium excretion and flow rate. Female Wistar rats were anaesthetised and infused intravenously with physiological saline at a rate of 4 mL/h via a jugular vein cannula. After 60 min, the infusion was switched to saline that contained D-Phe (200 mM) and, after 120 min, to saline that contained L-Phe (200 mM). The maximum plasma amino acid concentration observed under the conditions of these experiments was approximately 2 mM (baseline level around 0.05 mM) and the urine amino acid concentration rose to around 20 mM in the case of D-Phe and around 10 mM in the case of L-Phe. The data are means ± SEM (n=4).
1. Brown EM, Pollak M, Seidman CE, Seidman JG, Riccardi D, Hebert, SC. Calcium-ion-sensing cell-surface receptors. N. Engl. J. Med. 1995; 333: 234-40.
2. Brown EM, MacLeod RJ. Extracellular calcium sensing and extracellular calcium signaling, Physiol. Rev. 2001; 81: 239-97.
3. Nemeth EF, Steffey ME, Hammerland LG, Hung BCP, Vanwagenen BC, Delmar EG, Balandrin, MF. Calcimimetics with potent and selective activity on the parathyroid calcium receptor, Proc. Natl. Acad. Sci. USA 1998; 95: 4040-5.
4. Hauache OM, Hu J, Ray K, Xie R, Jacobson KA, Spiegel AM. Effects of a calcimimetic compound and naturally activating mutations on the human Ca2+ receptor and on Ca2+ receptor/metabotropic glutamate chimeric receptors, Endocrinology 2000; 141: 4156-63.
5. Conigrave AD, Quinn SJ, Brown EM. L-amino acid sensing by the extracellular Ca2+-sensing receptor, Proc. Natl. Acad. Sci. USA 2000; 97: 4814-9.
6. Zhang Z, Qiu W, Quinn SJ, Conigrave AD, Brown EM, Bai M. Three adjacent serines in the extracellular domains of the CaR are required for L-amino acid-mediated potentiation of receptor function, J. Biol. Chem. 2002; 277: 33727-35.
7. Ward DT, Riccardi D. Renal physiology of the extracellular calcium-sensing receptor, Pflügers Arch. 2002; 445: 169-76.
8. Riccardi D, Traebert M, Ward DT, Kaissling B, Biber J, Hebert SC, Murer H. Dietary phosphate and parathyroid hormone alter the expression of the calcium-sensing receptor (CaR) and the Na+-dependent Pi transporter (NaPi-2) in the rat proximal tubule, Pflügers Arch. 2000; 441: 379-87.
9. Ba J, Brown D, Friedman PA. Calcium-sensing receptor regulation of PTH-inhibitable proximal tubule phosphate transport, Am. J. Physiol. 2003; 285: F1233-F1243
10. Sands JM, Naruse M, Baum M, Jo I, Hebert SC, Brown EM, Harris HW. Apical extracellular calcium/polyvalent cation-sensing receptor regulates vasopressin-elicited water permeability in rat kidney inner medullary collecting duct, J. Clin.Invest. 1997; 99: 1399-405.
11. Peters JH. The determination of creatinine and creatine in blood and urine with the photoelectric colorimeter, J. Biol. Chem. 1942; 146: 179-86.
12. Jones BN, Paabo S, Stein S. Amino acid analysis and enzymatic sequence determination of peptides by an improved o-phthaldialdehyde precolumn labeling procedure, J. Liquid Chromatography 1981; 4: 565-86.
13. Fox J, Lowe SH, Petty BA, Nemeth EF. NPS R-568: a type II calcimimetic compound that acts on parathyroid cell calcium receptor of rats to reduce plasma levels of parathyroid hormone and calcium, J. Pharmacol. & Exp. Therapeut. 1999; 290: 473-9.