




|
Contents



|
1. There is strong evidence that the renal medullary circulation plays a key role in long-term blood pressure control. This, and evidence implicating sympathetic overactivity in development of hypertension, provides the need for understanding how sympathetic nerves affect medullary blood flow (MBF).
2. The precise vascular elements that regulate MBF under physiological conditions are unknown, but likely include the outer medullary portions of descending vasa recta, and afferent and efferent arterioles of juxtamedullary glomeruli, all of which receive dense sympathetic innervation.
3. Many early studies of the impact of sympathetic drive on MBF were flawed, both because of the methods used for measuring MBF, and because single and often intense neural stimuli were tested.
4. Recent studies have established that MBF is less sensitive than cortical blood flow (CBF) to electrical renal nerve stimulation, particularly at low stimulus intensities. Indeed, MBF appears to be refractory to increases in endogenous renal sympathetic nerve activity within the physiological range in all but the most extreme cases.
5. Multiple mechanisms appear to operate in concert to blunt the impact of sympathetic drive on MBF, including counter-regulatory roles of nitric oxide, and perhaps even paradoxical angiotensin II-induced vasodilatation. Regional differences in the geometry of glomerular arterioles are also likely to predispose MBF to be less sensitive than CBF to any given vasoconstrictor stimulus.
6. Failure of these mechanisms would promote reductions in MBF in response to physiological activation of the renal nerves, which could in turn lead to salt and water retention and hypertension.
‘Neural control of the capillary circulation in specific regions of the kidney has not been adequately studied’. This statement from Pomeranz et al. in 19681 could reasonably have been made almost 30 years later, with little progress being made in this field in the intervening period. However, renewed activity in this area since 19952 has increased our understanding of the influence of renal sympathetic drive on regional kidney blood flow. As we will describe in this review, there is now strong evidence that medullary blood flow (MBF) is less sensitive than cortical blood flow (CBF) to increases in renal sympathetic drive within the physiological range. This has important implications for the control of renal function, and in particular, the long-term regulation of arterial pressure. Nitric oxide appears to play a critical role in protecting the renal medulla from ischaemia due to renal nerve activation, but is not the only factor involved. For example, unique structural aspects of the medullary circulation probably contribute, and angiotensin II may have a surprising role as a counter-regulatory vasodilator within the medullary microcirculation. There is also the potential for neurochemical differences between nerves innervating vascular elements controlling MBF and CBF to contribute.
The aim of this review is to examine the mechanisms, and implications, of the neural regulation of MBF. However, we must first discuss three important issues: the unique vascular architecture of the kidney that underlies the differential control of MBF and CBF, the physiological imperatives of precise regulation of MBF, and the nature of the renal sympathetic innervation and its role in blood pressure control. We will then consider the evidence of differential neural control of CBF and MBF, and the potential mechanisms that underlie it.
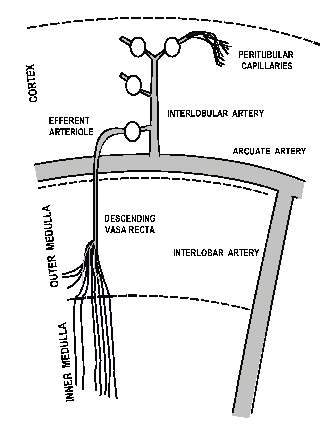
Figure 1. Schematic diagram of the architecture of the renal vasculature, and the extent of renal innervation to various vascular elements (shaded). Based on original figures by others3,71. Innervation data adapted from Barajas & Powers24.
The blood supply to the renal medulla arises from the efferent arterioles of juxtamedullary glomeruli, which comprise ∼10% of all glomeruli in the kidney (Figure 1). Thus, while all blood flow to the kidney enters the renal cortex, only ∼10% of this enters the renal medulla. In rats and dogs, reliable estimates of regional kidney blood flow have ranged from 2.6-7.4 ml/min/g in the cortex, 1.3-3.2 ml/min/g in the outer medulla, and 0.2-5.9 ml/min/g in the inner medulla3. Although these estimates show considerable variability between various studies (and species), it is widely regarded that blood flow per unit tissue weight in the outer and inner medulla is approximately 40% and 10%, respectively, that in the cortex. The maintenance of a relatively low MBF appears to be critical for maintaining the cortico-medullary solute gradient, and so urinary concentrating mechanisms3. On the other hand, because the renal medulla is a hypoxic environment even under normal conditions, there must be some trade off in the control of MBF, between maintenance of the cortico-medullary solute gradient (and so normal tubular function), and the supply of oxygen within the renal medulla (Figure 2). As will be described in detail below (see MBF and blood pressure control), there is also strong evidence that the level of MBF is a key factor in long-term control of blood pressure.
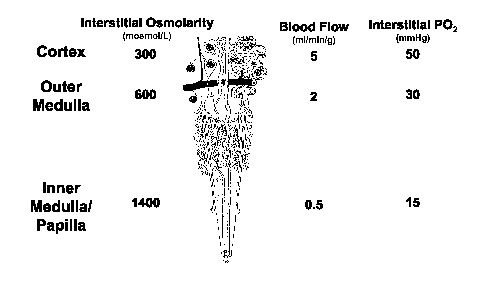
Figure 2. The trade-off between maintenance of the cortico-medullary solute gradient and medullary hypoxic damage. Diagram of the renal vasculature adapted from Beeuwkes & Bonventre73. Data relating to interstitial osmolarity, blood flow and interstitial PO2 compiled from Vander74, Pallone et al.3, and Lübbers & Baumgärtl75 respectively.
The precise vascular elements that regulate MBF under physiological conditions remain unknown. However, from a theoretical perspective changes in vascular resistance in juxtamedullary arterioles, or in downstream vascular elements within the medulla itself (eg, outer medullary descending vasa recta), could lead to large changes in MBF without significant alterations in total CBF. On the other hand, because juxtamedullary afferent arterioles arise near the origin of interlobular arteries (Figure 1), changes in interlobular artery calibre would be expected to impact less on MBF (and juxtamedullary cortical blood flow) than on the bulk of CBF.
Heterogeneity of the geometry of glomerular arterioles may also contribute to the differential regulation of CBF and MBF. Afferent and (particularly) efferent arterioles of juxtamedullary glomeruli have considerably greater calibre than their counterparts in the mid- and outer-cortex4 (Figure 1). Because vascular resistance is inversely proportional to vessel radius to the power of 4, comparable changes in vessel radius result in lesser absolute change in vascular resistance in the larger juxtamedullary arterioles, than in their counterparts in other regions of the cortex4.
Although the precise aetiology of essential hypertension remains unknown, there is persuasive evidence that the initial trigger resides within the kidney5. One line of evidence in support of this notion arose from the seminal work of Guyton and colleagues6, showing that the pressure diuresis/natriuresis mechanism provides a ‘non-adapting’ feedback system by which arterial pressure can be controlled in the long-term. The relationship, between renal perfusion pressure and salt and water excretion (pressure diuresis/natriuresis), is set at higher pressures in all forms of hypertension that have been studied, and hypertension can be ameliorated by treatments that restore this relationship towards normal. Another important line of evidence comes from studies of renal transplantation between hypertensive and normotensive subjects. Both in rats and humans, there is good evidence that ‘the blood pressure follows the kidney’7. That is, when a kidney from a normotensive subject is transplanted into a hypertensive subject, arterial pressure falls. Conversely, when the kidney from a hypertensive subject, or a normotensive subject genetically pre-disposed to hypertension, is transplanted into a normotensive subject, hypertension develops.
In a series of elegant studies reviewed in detail previously8-11, Cowley, Roman, Mattson and colleagues have provided persuasive evidence that MBF is a critical factor in the long-term control of arterial pressure. They have utilised a conscious rat model in which CBF and MBF are measured chronically using implanted optical fibres, while vasoactive agents are administered directly into the renal medulla. Chronic medullary interstitial infusion of vasoconstrictors, at doses that reduce MBF, produce hypertension, whereas similar infusions of vasodilators that increase MBF can ameliorate hypertension. This effect seems to be mediated through alterations in the pressure diuresis/natriuresis relationship, which is shifted to higher pressures by both chronic and acute medullary interstitial infusions of vasoconstrictors, and shifted to lower pressures by medullary interstitial infusions of vasodilators. We have confirmed some of these observations in a different species, showing that acute medullary intersitital (but not intravenous) infusion of noradrenaline shifts the pressure diuresis/natriuresis relationship to higher pressures in anaesthetized rabbits12,13.
The precise mechanisms by which reductions in MBF shift the pressure diuresis/natriuresis relationship to higher pressure remain a matter of controversy. Cowley and colleagues have developed the hypothesis, for which there is considerable experimental support,8-11 that increases in MBF in response to increased renal perfusion pressure actually mediate pressure diuresis/natriuresis. Increased vasa recta capillary hydrostatic pressure (secondary to increased vasa recta blood flow) will result in increased medullary interstitial hydrostatic pressure, which will be transmitted throughout the kidney because of the low compliance of the kidney due to the presence of the renal capsule. Increased renal interstitial hydrostatic pressure reduces sodium reabsorption in a number of segments of the nephron, probably in part through enhanced back-leak along paracellular pathways. However, the integrity of this hypothesis depends on the idea that MBF, unlike total renal blood flow (RBF) and CBF, is relatively poorly autoregulated. The degree to which MBF is autoregulated remains a matter of controversy,14,15 probably in part because of limitations in available methods for estimating MBF.
There is clear evidence that renal sympathetic drive is increased during the development of hypertension both in the spontaneously hypertensive rat (SHR) and in human essential hypertension. Thus, basal post-ganglionic sympathetic nerve activity16, and emotional stress-induced increases in post-ganglionic sympathetic nerve activity and reductions in sodium excretion17, are enhanced in SHR compared with normotensive Wistar Kyoto control rats (WKY). Furthermore, renal sympathetic drive, as measured by noradrenaline spillover, is also increased in human essential hypertension18. Increased renal sympathetic drive appears to contribute to the pathogenesis of hypertension, since in SHR chronic bilateral renal denervation, achieved by repeated denervation between weeks 4 and 16 after birth, blocks 30-40% of the expected progressive elevation of arterial pressure19. A similar regimen of bilateral renal denervation in WKY has no effect on arterial pressure19.
The precise mechanisms by which increased renal sympathetic drive contributes to the pathogenesis of hypertension remain unknown. The fact that reductions in MBF can shift the pressure diuresis/natriuresis relationship to higher pressures (right-ward shift), which if maintained chronically produces hypertension, provides the impetus for our interest in the neural control of MBF.
The origin of the efferent sympathetic innervation of the kidney differs among species, but in general arises from multiple ganglia of the celiac plexus, the lumbar splanchnic nerve and the intermesenteric plexus20. Post-ganglionic nerves enter the kidney in association with the renal vasculature, and follow the course of the renal arterial tree as it branches to form interlobar, arcuate, and interlobular arteries. These neurones in turn innervate the afferent and efferent arterioles, and the outer medullary portions of descending vasa recta, but not vascular elements within the inner medulla and papilla21 (Figure 1). Consistent with these anatomical observations, juxtamedullary afferent and efferent arterioles of the rat hydronephrotic kidney constrict in response to renal nerve stimulation22.
Previous studies of regional differences in innervation density within the kidney indicate that juxtamedullary afferent and efferent arterioles, and their associated outer medullary descending vasa recta, are densely innervated. For example, McKenna & Angelakos found the juxtamedullary cortex and outer medulla to have the greatest concentration of noradrenaline within the dog kidney, levels being ∼40-60% less in the mid- and subcapsular-cortex, and low in the inner medulla23. Barajas and Powers provided more direct evidence of dense juxtamedullary vascular innervation, using autoradiography to detect uptake of exogenous [3H]-noradrenaline (presumptively by sympathetic nerves) in rat kidney24. They found greater density of autoradiographic grains on afferent, compared with efferent arterioles throughout the cortex, but autoradiographic grain density was similar in each of these vascular elements in the outer- mid- and juxtamedullary cortex. Quantitative analysis of the innervation density of outer medullary descending vasa recta was not included in their study, although the amount of autoradiographic grains overlapping the vasculature was greater in the outer stripe of the outer medulla than in any other kidney region. It must be born in mind, however, that the techniques that have been applied to this problem have considerable limitations. Most evidence suggests that sympathetic neurotransmission in blood vessels occurs chiefly via specialised neuromuscular junctions, at which varicosities form a close contact (< 100 nm) with arteriolar smooth muscle cells25. In the rabbit kidney, ∼80% of sympathetic varicosities within the arteriolar region form these specialised neuromuscular junctions26. On the other hand, it has also been argued that sympathetic neurotransmitters can also act at some distance from their site of release within the kidney, particularly in the control of tubular function20. Nevertheless, the relative distribution of specialised neuromuscular junctions in vascular elements controlling CBF and MBF would better reflect the density of ‘functional’ sympathetic innervation in the renal vasculature, than measures of tissue noradrenaline content per se23, or the density of sites of noradrenaline uptake24. There is a need, therefore, for further detailed studies of the innervation of vascular elements controlling MBF and CBF.
Most evidence suggests that the predominant neurotransmitter in renal sympathetic nerves is noradrenaline. Thus, while dopamine also appears to be present in these nerves as a precursor of noradrenaline synthesis, there is little compelling evidence of specific dopaminergic nerves within the kidney20. Moreover, while acetylcholine is found within the kidney, it appears not to be associated with renal nerves20. Nevertheless, there is now strong evidence that co-transmitters, including neuropeptide Y and ATP participate in renal sympathetic neurotransmission20 and partially mediate renal nerve stimulation induced-reductions in global RBF27-30. Other neurotransmitters, including vasoactive intestinal polypeptide and neurotensin, have been identified within the renal vasculature31, and galanin has been identified in a proportion of the neurons innervating the kidney32. Their roles in renal sympathetic neurotransmission and in regulating renal function remain to be determined. Neuropeptide Y31 and its binding sites33, and also neurotensin and vasoactive intestinal polypeptide31 have been localised to vascular elements of the medullary circulation (including juxtamedullary afferent and efferent arterioles), raising the possibility that these sympathetic co-transmitters could contribute to the neural control of MBF.
All available methods for estimating regional kidney blood flow have limitations that must be considered in the interpretation of experimental data3,34. Methods used in early studies of the control of MBF, based on para-aminohippuric acid clearance, washout of diffusible tracers such as 85Kr, H2, and heat (thermodilution), renal extraction of diffusible indicators such as 42K and 86Rb, indicator transit time, albumin accumulation and microspheres have been shown to be (more or less) invalid from either practical or theoretical standpoints3,33. For the most part, these methods are also limited by the fact that they do not provide ‘real time’ measurements of blood flow in individual animals. A further limitation of many early studies of the neural control of intrarenal blood flow is that they often employed single, intense stimuli, well beyond what one might consider to be physiologically relevant. However, it is worthwhile for us to briefly consider the results of studies using these techniques, because they allow us to appreciate both the heroic efforts of earlier investigators, and the evolution of our understanding, of the neural control of intrarenal blood flow.
Trueta et al. were the first to study this issue (in 1947), using the intrarenal distribution of injected radiocontrast material and Indian ink as markers of blood flow in anaesthetized rabbits35. Their observations were entirely qualitative, but prophetic, in that they suggested that renal nerve stimulation induced redistribution of blood flow from the outer cortex to the inner cortex and medulla. In contrast, Houck (in 1951), who also used the Indian ink distribution method in anaesthetized dogs, to study the effects of intense electrical stimulation of the renal nerves, concluding that CBF and MBF were similarly dramatically decreased by intense renal nerve stimulation36. Similar conclusions were drawn by Aukland in 1968, using a method for determining local H2 gas clearance within the outer medulla in anaesthetized dogs. They found that total RBF and outer cortical H2 gas clearance both fell by ∼40% during intense renal nerve stimulation, but also conceded that ‘due to the counter current exchange of gas between ascending and descending vasa recta, the clearance is not necessarily linearly related to blood flow’37. Similar observations, using a similar technique in anesthetized rats, were reported by Chapman et al. in 198238. Thus, with the exception of the initial study by Trueta et al., the unanimous conclusion from the studies described above was that CBF and MBF are similarly sensitive to the effects of activation of the renal sympathetic nerves.
Some studies were performed in which graded neural stimuli were applied, but the picture arising from them was far from clear. Pomeranz et al. (1968) used the 85Kr autoradiography technique in both anaesthetized and conscious dogs, and concluded that although intense renal nerve activity reduced both CBF and MBF, mild stimulation of the renal nerves actually increased MBF1. In almost direct contrast, Hermansson et al. reported their study using 86Rb uptake in anaesthetized rats in 1984, concluding that MBF was more sensitive than CBF to the ischaemic effects of low frequency renal nerve stimulation39. These observations are clearly at odds with the results of more recent studies using laser Doppler flowmetry.
At present, the most widely used method for estimation of blood flow in specific regions of the kidney is laser Doppler flowmetry. This technique has the advantage that measurements can be made in real-time, and in anatomically specific regions of the kidney. There is good evidence of a direct relationship between laser Doppler flux and erythrocyte velocity both in model systems in vitro15,40-42, and in the kidney in vivo15,40,43,44. However, it must also be recognised that in highly perfused tissues such as the kidney, laser Doppler flux is relatively insensitive to changes in the volume fraction of red blood cells in the tissue15,40,41. Therefore, changes in MBF due to changes in the number of perfused capillaries (capillary recruitment) are unlikely to be detected by laser Doppler flowmetry. Nevertheless, this method does represent a considerable technical breakthrough in the study of regional kidney blood flow. Over the last decade, studies from a number of separate research groups using this technique have led to the unequivocal conclusion that MBF is relatively insensitive to renal sympathetic drive, especially at stimulus intensities within the physiological range.
Rudenstam et al. showed that graded renal nerve stimulation (2-5 Hz at 5 V and 1 ms duration) in anaesthetized rats produced progressive reductions in RBF and CBF, but only small changes in blood flow in the renal papilla (the very inner part of the medulla)2. We subsequently performed similar studies in anaesthetized rabbits, showing that in this species inner MBF was reduced in a progressive fashion by graded (frequency or amplitude) renal nerve stimulation, but that MBF was reduced less than RBF or CBF, particularly at stimulation frequencies of 3 Hz or less45 (Figure 3). Collectively, these studies suggested that the medullary circulation is relatively insensitive to the ischaemic effects of renal sympathetic drive, but also raised the possibility that some regional differences in sensitivity might exist within the medulla. To investigate this latter possibility, we tested the effects of graded renal nerve stimulation on laser Doppler blood flow measurements at 2 mm intervals from the surface of the cortex to close to the tip of the papilla42. We found that responses to renal nerve stimulation in the renal cortex (≤ 3 mm below the kidney surface) were always greater than those within the medulla (≥ 5 mm below the kidney surface), but that responses within the inner and outer medulla were indistinguishable. Thus, while these data confirm that renal nerve activation can differentially affect CBF and MBF, they do not support the notion that it can differentially affect perfusion at different levels of the medulla.
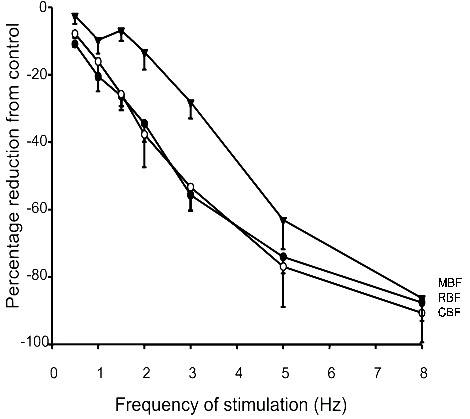
Figure 3. Mean responses of total renal blood flow (RBF, ●), and laser Doppler flowmetry measurements of cortical blood flow (CBF, ○) and medullary blood flow (MBF,▼), to graded frequencies of renal nerve stimulation (supramaximal voltage, 2 ms duration) in anaesthetized rabbits. Symbols represent mean ± s.e.mean of observations in 8 rabbits. Note that analysis of variance showed that, across all frequencies of electrical stimulation, responses of MBF differed from those of RBF and CBF (P < 0.001). In contrast, responses of RBF and CBF were indistinguishable (P > 0.05). Redrawn from Leonard et al.45
Electrical stimulation of the renal sympathetic nerves is a useful technique for producing graded increases in renal sympathetic drive, but it does not mimic naturally occurring renal sympathetic nerve activity (RSNA)46. Endogenous RSNA has a bursting pattern, with the amplitude of each burst probably largely reflecting the recruitment of individual axons47. In most cases, reflex changes in RSNA mainly reflect changes in the amplitude of bursts, rather than changes in their frequency48,49. Relating the frequency of electrical stimulation to changes in endogenous RSNA is therefore problematic46. Given this caveat, we can at least say that similar reductions in CBF of ∼20% are achieved in anaesthetized rabbits with 1 Hz electrical stimulation45, and a hypoxic stimulus that increases RSNA by ∼80%50. Therefore, our observation that the relative insensitivity of MBF to renal nerve stimulation is most clearly seen at low frequencies of stimulation raises the possibility that MBF might be refractory to the basal level of RSNA, and to reflex increases in RSNA associated with physiological manoeuvres that reduce RBF. This does indeed seem to be the case. For example, CBF but not MBF is reduced by arterial chemoreceptor stimulation in conscious rats51 and hypoxia in anaesthetized rabbits50 (Figure 4). Furthermore, while hypotensive haemorrhage consistently reduces CBF, MBF has been observed to either remain unchanged or to increase52,53, or to be reduced less than CBF54-56. Conversely, renal denervation in anaesthetized rats increases CBF but not MBF57.
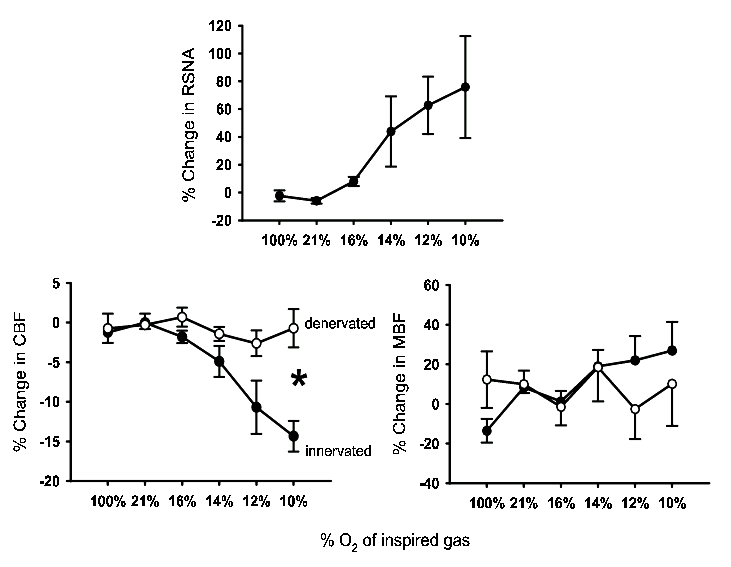
Figure 4. Responses of renal sympathetic nerve activity, and laser Doppler measurements of cortical blood flow (CBF) and medullary blood flow (MBF) to progressive hypoxia. Experiments were performed in anaesthetized, artificially ventilated rabbits, and hypoxaemia was induced by exposure to progressively hypoxic gas mixtures. Responses of CBF and MBF were determined in rabbits with intact renal nerves (●; n = 7) and in rabbits in which the renal nerves were destroyed (○; n = 6). Symbols and error bars represent mean ± s.e.mean. * P < 0.05 for interaction term between ‘state’ (intact or denervated) and the response to progressive hypoxia, from analysis of variance. Data redrawn from Leonard et al.50
On the other hand, MBF does not appear to be entirely insensitive to reflex increases in RSNA. Evoking the nasopharyngeal reflex in conscious rabbits, by exposure to cigarette smoke, transiently increased RSNA by ∼135%48. This reflex is accompanied by little change in arterial pressure, but falls in cardiac output, RBF, CBF and MBF are observed58 (Figure 5). Indeed, MBF and CBF were reduced similarly by the nasopharyngeal reflex in conscious rabbits, which seems at odds with the notion that MBF is less sensitive than CBF to reflex increases in RSNA. An explanation for this paradox might lie in differences between the dynamic responses of CBF and MBF to neural activation. In particular, MBF seems able to respond faster to renal sympathetic activation59, and to be more sensitive than CBF or total RBF to oscillations in RSNA at frequencies normally present in endogenous RSNA45. This might increase the relative responsiveness of MBF to transient increases in RSNA associated with manoeuvres such as the nasopharyngeal reflex. The mechanistic and anatomical bases of the differing frequency response characteristics of CBF and MBF remain unknown.
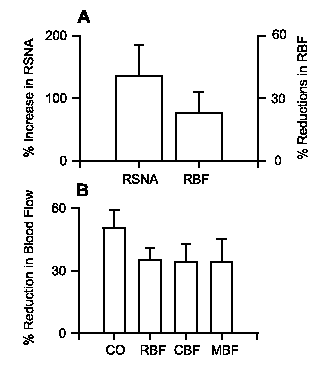
Figure 5. Responses in conscious rabbits of renal sympathetic nerve activity (RSNA), renal blood flow (RBF), cardiac output (CO), cortical blood flow (CBF) and medullary blood flow (MBF) to exposure to cigarette smoke (the nasopharyngeal reflex). Panel A represents the results of a study in rabbits equipped for simultaneous measurement of RSNA and RBF in the left kidney48. Note that changes in RSNA and RBF are shown on different scales. Panel B shows the results of an experiment in rabbits equipped for simultaneous measurement of CO, and RBF, CBF and MBF in the left kidney58. The reflex comprises transient reductions in heart rate, CO and RBF that usually reach a maximum within the first 5 s after exposure to smoke. Data represent the mean ± s.e.mean (n = 8-12) of maximum changes from control. Note that responses of RBF in the two experiments are comparable, and that both CBF and MBF are reduced by this reflex which more than doubles RSNA.
Because MBF is refractory to mild to moderate increases in RSNA, it seems likely that the renal nerves play little role in its physiological regulation. However, in pathological conditions such as heart failure, where RSNA can increase by over 200%60, MBF might be chronically reduced, which would exacerbate salt and water retention. Furthermore, MBF might also be chronically reduced if its sensitivity to RSNA were somehow increased, perhaps through failure of mechanisms protecting the medulla from the ischaemic effects of sympathetic activation. Potentially, this could lead to the development of hypertension. Much of our recent research, therefore, has focussed on elucidating the mechanisms underlying the relative insensitivity of MBF to renal sympathetic drive. From a theoretical perspective, a number of potential mechanisms could contribute, which are discussed separately below.
As discussed earlier, (see The renal medullary circulation: structure and function), the fact that juxtamedullary afferent and (particularly) efferent arterioles have greater calibre than their counterparts in other regions of the kidney, should theoretically predispose MBF to be less sensitive than the bulk of CBF to virtually all vasoconstrictor stimuli. In support of this notion, we have found that while some vasoconstrictors preferentially reduce MBF more than CBF (eg vasopressin peptides), most reduce CBF more than MBF (eg RSNA, angiotensin II, endothelin peptides)58,61-67. Furthermore, renal arterial infusions of angiotensin II4 and endothelin-166 constrict juxtamedullary afferent and efferent arterioles similarly to their counterparts in other regions of the kidney (determined by vascular casting methods), yet MBF is little affected by these agents in the face of large changes in total RBF and CBF65,66. It seems likely, therefore, that the vascular architecture of the kidney is arranged in a way that protects the medulla from the ischaemic effects of a range of vasoconstrictor stimuli, including sympathetic nerve activation.
As previously mentioned (see Innervation of vascular elements controlling MBF), available evidence suggests that juxtamedullary glomerular arterioles and outer medullary descending vasa recta are richly innervated, so this seems unlikely to account for the relative insensitivity of MBF to sympathetic activation. However, more detailed information at the ultrastructural level, regarding the density of neuromuscular junctions on the various vascular elements within the kidney, is required before this potential mechanism can be completely excluded.
We recently tested the effects of blockade of α1-adrenoceptors on regional kidney blood flow responses to renal nerve stimulation68. As expected, the α1-adrenoceptor antagonist prazosin greatly blunted responses of RBF and CBF to renal nerve stimulation, but to our surprise, had no detectable effect on responses of MBF. We can exclude roles for α2-adrenoceptors in mediating the post-junctional response to renal nerve stimulation, because the α2-adrenoceptor antagonist rauwolscine did not inhibit responses of MBF to renal nerve stimulation. These observations raise the interesting possibility that sympathetic co-transmitters make an important contribution to mediating the effects of sympathetic nerve activity on MBF.
The role of the vascular endothelium in modulating responses to vasoactive factors is well established10. More recently, it has become clear that such factors are also released from the tubular epithelium, and that so-called ‘tubulovascular cross-talk’ plays a key role in the regulation of renal vascular tone10. Previous studies of the contribution of these mechanisms to the neural control of regional kidney blood flow have, for the most part, relied on intravascular administration of noradrenaline as a surrogate for neural noradrenaline release. Such experiments must be interpreted with care, since noradrenaline infusion does not adequately mimic sympathetic nerve activation, which likely involves neurotransmitter (including co-transmitter) release at specialised neuromuscular junctions25. Nevertheless, these experiments have provided important mechanistic information that has formed the basis of our research in this area.
The relative insensitivity of MBF to noradrenaline infusions (intravenous or renal arterial) appears to be largely due to nitric oxide release61,69. Our recent results suggest that a similar mechanism might operate to protect the medulla from the ischaemic effects of sympathetic nerve activation, since blockade of nitric oxide synthesis62 enhances MBF responses to renal nerve stimulation in rabbits. However, even after nitric oxide synthase blockade, renal nerve stimulation still reduces MBF less than CBF62, indicating that other mechanisms also contribute to the relative insensitivity of the medullary circulation to sympathetic activation. Prostanoids appear to have little net role in modulating renal vascular responses to activation of the sympathetic nerves, as the cyclooxygenase inhibitor ibuprofen did not significantly affect responses of RBF, CBF or MBF to renal nerve stimulation in anaesthetized rabbits70. However, we also recently found that under conditions of prior cyclooxygenase blockade, nitric oxide synthase blockade did not enhance the response of MBF to renal nerve stimulation70. These observations contrast directly with those of our previous study under conditions of intact cyclooxygenase activity62, and raise the intriguing possibility, that the impact of nitric oxide synthase blockade on responses of MBF to renal nerve stimulation, are at least partly mediated through vasoconstrictor products of cyclooxygenase.
We recently obtained evidence that circulating hormones such as angiotensin II and arginine vasopressin could play a key role in determining the nature of the regional renal haemodynamic response to increased renal sympathetic drive63. For example, angiotensin II is known to act at a number of levels to enhance sympathetic neurotransmission71, but this endocrine/paracrine/autocrine hormone also has a unique action within the medullary circulation, in that it can induce vasodilation through activation of AT1-receptors, and subsequent release of nitric oxide and vasodilator prostaglandins10,61,64,65. To test whether angiotensin II might differentially affect responses to sympathetic activation in the medullary and cortical circulations, we tested the effects, on responses to renal nerve stimulation, of renal arterial infusion of angiotensin II in anaesthetized rabbits, at a dose that slightly reduced basal RBF and CBF but did not significantly affect basal MBF63. We found that the angiotensin II infusion virtually abolished reductions in MBF induced by renal nerve stimulation, without affecting responses of RBF and CBF (Figure 6). Thus, elevated intrarenal levels of angiotensin II appear to selectively inhibit renal nerve stimulation-induced ischaemia in the medullary circulation. The physiological significance of this phenomenon, and the mechanisms mediating it, remain to be determined.
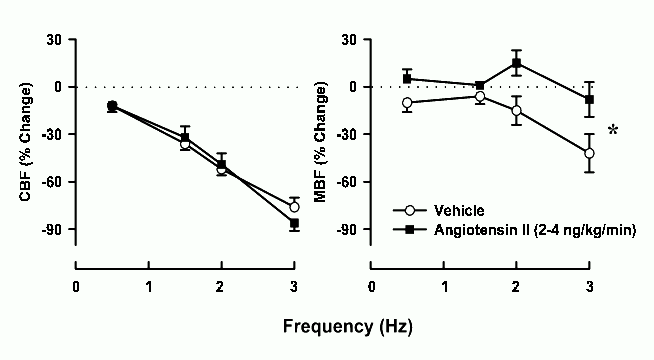
Figure 6. Responses of cortical blood flow (CBF) and medullary blood flow (MBF) to renal nerve stimulation in anaesthetized rabbits receiving a renal arterial infusion of isotonic saline (○), or angiotensin II (2-4 ng/kg/min, ■) (n = 9). Saline infusion did not significantly affect baseline CBF or MBF, whereas angiotensin II infusion significantly reduced baseline CBF (by 14 ± 5%) but not MBF. * P < 0.05 for significant difference, across all frequencies, in the responses to renal nerve stimulation during angiotensin II infusion, compared with the responses during saline infusion. Data redrawn from Guild et al.63
There is now strong evidence that activation of the renal sympathetic nerves has less impact on MBF than CBF, particularly at moderate stimulus intensities. Indeed, the medullary circulation appears to be refractory to basal levels of endogenous sympathetic nerve activity, and to all but the most profound reflex increases in sympathetic drive. The precise nature of the mechanisms that limit the sensitivity of MBF to sympathetic drive remain unknown, although recent experiments suggest roles for nitric oxide and possibly angiotensin II. It also seems likely that regional differences in the geometry of glomerular arterioles pre-disposes MBF to respond less than CBF to any given vasoconstrictor stimulus (Figure 7). Other mechanisms, including the potential for roles of sympathetic co-transmitters, require investigation.
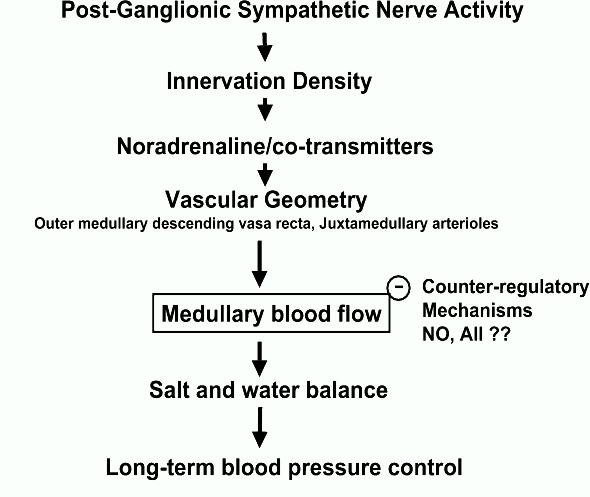
Figure 7. Working hypothesis of the factors underlying the relative insensitivity of renal medullary blood flow (MBF) to renal sympathetic drive. Responses of MBF to sympathetic activation will depend on the level of post-ganglionic sympathetic nerve activity, the functional density of the sympathetic innervation of vascular elements controlling MBF, on the nature of neurotransmission in these neurones, and on the basal calibre of vascular elements controlling MBF relative to those in the bulk of the renal cortex. Nitric oxide (NO), and perhaps also circulating angiotensin II (AII), seem to play key roles in blunting responses of MBF to renal nerve stimulation. Failure of these mechanisms could lead to salt and water retention under conditions of sympatho-adrenal activation, and so the development of hypertension.
Dysfunction of the mechanisms that protect the medullary circulation from ischaemia due to activation of the renal nerves would increase the sensitivity of MBF to renal sympathetic drive. This could potentially lead to chronic reductions in MBF, salt and water retention, and the subsequent development of hypertension (Figure 7). Future studies should aim to directly test this hypothesis, and determine whether neurally-mediated reductions in MBF contribute to the development of essential hypertension, and also to salt and water retention in pathological conditions associated with increased sympathetic drive, such as heart failure.
Original studies by the authors described in this article were supported by grants from the National Health and Medical Research Council of Australia (143603, 143785), the National Heart Foundation of Australia (G 98M 0125), the Ramaciotti Foundations (A 6370, RA 159/98), the Marsden Fund of New Zealand, and the Auckland Medical Research Foundation.
1. Pomeranz BH, Birtch AG, Barger AC. Neural control of intrarenal blood flow. Am. J. Physiol. 1968; 215: 1067-1081.
2. Rudenstam J, Bergström G, Taghipour K, Göthberg G, Karlström G. Efferent renal sympathetic nerve stimulation in vivo. Effects on regional renal haemodynamics in the Wistar rat, studied by laser-Doppler technique. Acta Physiol. Scand. 1995; 154: 387-394.
3. Pallone TL, Robertson CR, Jamison RL. Renal medullary microcirculation. Physiol. Rev. 1990; 70: 885-920.
4. Denton KM, Anderson WP, Sinniah R. Effects of angiotensin II on regional afferent and efferent arteriole dimensions and the glomerular pole. Am. J. Physiol. 2000; 279: R629-R638.
5. Luke RG. Essential hypertension: a renal disease? A review and update of the evidence. Hypertension 1993; 21: 380-390.
6. Guyton AC. Long-term arterial pressure control: an analysis from animal experiments and computer and graphic models. Am. J. Physiol. 1990; 259: R865-R877.
7. Rettig R, Schmitt B, Peilzl B, Speck T. The kidney and primary hypertension. Contributions from renal transplantation studies in animals and humans. J. Hypertension 1993; 11: 883-891.
8. Roman RJ, Zou A-P. Influence of the renal medullary circulation on the control of sodium excretion. Am. J. Physiol. 1993; 265: R963-R973.
9. Cowley AWJr. Role of the renal medulla in volume and arterial pressure regulation. Am. J. Physiol. 1997; 273: R1-R15.
10. Cowley AWJr, Mori T, Mattson D, Zou A-P. Role of renal NO production in the regulation of medullary blood flow. Am. J. Physiol. 2003; 284: R1355-R1369.
11. Mattson D. Importance of the renal medullary circulation in the control of sodium excretion and blood pressure. Am. J. Physiol. 2003; 284: R13-R27.
12. Correia AG, Bergström G, Lawrence AJ, Evans RG. Renal medullary interstitial infusion of norepinephrine in anesthetized rabbits: methodological considerations. Am. J. Physiol. 1999; 277: R112-R122.
13. Correia AG, Madden AC, Bergström G, Evans RG. Effects of renal medullary and intravenous norepinephrine on renal antihypertensive function. Hypertension 2000; 35: 965-970.
14. Majid DS, Said K, Omoro SA, Navar LG. Nitric oxide dependency of arterial pressure-induced changes in renal interstitial hydrostatic pressure in dogs. Circ. Res. 2001; 88: 347-351.
15. Eppel GA, Bergström G, Anderson WP, Evans RG. Autoregulation of renal medullary blood flow in rabbits. Am. J. Physiol. 2003; 284: R233-R244.
16. Lundin S, Ricksten S-E, Thoren P. Renal sympathetic activity in spontaneously hypertensive rats and normotensive controls, as studied by three different methods. Acta Physiol. Scand. 1984; 120: 265-272.
17. Lundin S, Thoren P. Renal function and sympathetic activity during mental stress in normotensive and spontaneously hypertensive rats. Acta Physiol. Scand. 1982; 115: 115-124.
18. Esler MG, Lambert G, Jennings G. Increased regional sympathetic nervous activity in human hypertension: causes and consequences. J. Hypertens. 1990; 8(Suppl 7): S53-S57.
19. Norman RA, Dzielak DJ. Role of renal nerves in onset and maintenance of spontaneous hypertension. Am. J. Physiol. 1982; 243: H284-H288.
20. DiBona GF, Kopp UC. Neural control of renal function. Physiol. Rev. 1997; 77: 75-197.
21. Barajas L, Liu L, Powers K. Anatomy of the renal innervation: intrarenal aspects and ganglia of origin. Can. J. Physiol. Pharmacol. 1992; 70: 735-749.
22. Chen J, Fleming JT. Juxtamedullary afferent and efferent arterioles constrict to renal nerve stimulation. Kid. Int. 1993; 44: 684-691.
23. McKenna OC, Angelakos ET. Adrenergic innervation of the canine kidney. Circ. Res. 1968; 22: 345-354.
24. Barajas L, Powers K. Monoaminergic innervation of the rat kidney: a quantitative study. Am. J. Physiol. 1990; 259: F503-F511.
25. Brock JA, Cunnane T. Electrophysiology of neuroeffector transmission in smooth muscle. In: Burnstock G, Hoyle CHV (eds). Autonomic Neuroeffector Mechanisms. Harwood Academic Publishers: Chur. 1992; pp 121-211.
26. Luff SE, Hengstberger SG, McLachlan EM, Anderson WP. Distribution of sympathetic neuroeffector junctions in the juxtaglomerular region of the rabbit kidney. J. Autonom. Nerv. Syst. 1992; 40: 239-254.
27. DiBona GF, Sawin LL. Role of neuropeptide Y in renal sympathetic vasoconstriction: studies in normal and congestive heart failure rats. J. Lab. Clin. Med. 2001; 138: 119-129.
28. Malmström RE, Balmér KC, Lundberg JM. The neuropeptide Y (NPY) Y1 receptor antagonist BIBP 3226: equal effects on vascular responses to exogenous and endogenous NPY in the pig in vivo. Br. J. Pharmacol. 1997; 121: 595-603.
29. Pernow J, Lundberg JM. Release and vasoconstrictor effects of neuropeptide Y in relation to non-adrenergic sympathetic control of renal blood flow in the pig. Acta Physiol. Scand. 1989; 136: 507-517.
30. Schwartz DD, Malik KU. Renal periarterial nerve stimulation-induced vasoconstriction at low frequencies is primarily due to release of a purinergic transmitter in the rat. J. Pharmacol. Exp. Ther. 1989; 250: 764-771.
31. Reinecke M, Forssmann WG. Neuropeptide (neuropeptide Y, neurotensin, vasoactive intestinal polypeptide, substance P, calcitonin gene-related peptide, somatostatin) immunohistochemistry and ultrastructure of renal nerves. Histochem. 1988; 89: 1-9.
32. Longley CD, Weaver LC. Proportions of renal and splenic postganglionic sympathetic populations containing galanin and dopamine beta hydroxylase. Neuroscience 1993; 55: 253-261.
33. Leys K, Schachter M, Sever P. Autoradiographic localisation of NPY receptors in rabbit kidney: comparison with rat, guinea-pig and human. Eur. J. Pharmacol. 1987; 134: 233-237.
34. Hansell P. Evaluation of methods for estimating renal medullary blood flow. Renal Physiol. Biochem. 1992; 15: 217-230.
35. Trueta J, Barclay AE, Daniel PM, Franklin KJ, Prichard MML. Studies of the Renal Circulation. 1947, Oxford, Blackwell Scientific Publications.
36. Houck CR. Alterations in renal hemodynamics and function in separate kidneys during stimulation of renal artery nerves in dogs. Am. J. Physiol. 1951; 167: 523-530.
37. Aukland K. Effect of adrenaline, noradrenaline, angiotensin and renal nerve stimulation on intrarenal distribution of blood flow in dogs. Acta Physiol. Scand. 1968; 72: 498-509.
38. Chapman BJ, Horn NM, Robertson MJ. Renal blood-flow changes during renal nerve stimulation in rats treated with α-adrenergic and dopaminergic blockers. J. Physiol. 1982; 325: 67-77.
39. Hermansson K, Öjteg G, Wolgast M. The cortical and medullary blood flow at different levels of renal nerve activity. Acta Physiol. Scand. 1984; 120: 161-169.
40. Roman RJ, Smits C. Laser-Doppler determination of papillary blood flow in young and adult rats. Am. J. Physiol. 1986; 251: F115-F124.
41. Almond NE, Wheatley AM. Measurement of hepatic perfusion in rats by laser Doppler flowmetry. Am. J. Physiol. 1992; 262: G203-G209.
42. Guild S-J, Eppel GA, Malpas SC, Rajapakse NW, Stewart A, Evans RG. Regional responsiveness of renal perfusion to activation of the renal nerves. Am. J. Physiol. 2002; 283: R1177-R1186.
43. Stern MD, Bowen PD, Parma R, Osgood RW, Bowman RL, Stein JH. Measurement of renal cortical and medullary blood flow by laser-Doppler spectroscopy in the rat. Am. J. Physiol. 1979; 236: F80-F87.
44. Fenoy FJ, Roman RJ. Effect of volume expansion on papillary blood flow and sodium excretion. Am. J. Physiol. 1991; 260: F813-F822.
45. Leonard BL, Evans RG, Navakatikyan MA, Malpas SC. Differential neural control of intrarenal blood flow. Am. J. Physiol. 2000; 279: R907-R916.
46. Malpas SC, Guild S-J, Evans RG. Responsiveness of the renal vasculature: relating electrical stimulation to endogenous nerve activity is problematic. Am. J. Physiol. 2003; 284: F594-F596.
47. Malpas SC. The rhythmicity of sympathetic nerve activity. Prog. Neurobiol. 1998; 56: 65-96.
48. Malpas SC, Evans RG. Do different levels and patterns of sympathetic activation all provoke renal vasoconstriction? J. Autonom. Nerv. Syst. 1998; 69: 72-82.
49. Malpas SC, Evans RG, Head GA, Lukoshkova EV. Contribution of renal nerves to renal blood flow variability during hemorrhage. Am. J. Physiol. 1998; 274: R1283-R1294.
50. Leonard BL, Malpas SC, Denton KM, Madden AC, Evans RG. Differential control of intrarenal blood flow during reflex increases in sympathetic nerve activity. Am. J. Physiol. 2001; 280: R62-R68.
51. Ledderhos C, Gross V, Cowley AWJr. Pharmacological stimulation of arterial chemoreceptors in conscious rats produces differential responses in renal cortical and medullary blood flow. Clin. Exp. Pharmacol. Physiol. 1998; 25: 536-540.
52. Brezis M, Heyman SN, Epstein FH. Determinants of intrarenal oxygenation II: Hemodynamic effects. Am. J. Physiol. 1994; 267: F1063-F1068.
53. Ganguli M, Tobian L. Hypertension from carotid occlusion decreases renal papillary blood flow, hypotension from hemorrhage increases it, an autoregulatory paradox. Hypertens. Res. 1996; 19: 17-22.
54. Fischer R, Ikeda S, Sarma JSM, Bing RJ. The effects of indomethacin, 6-hydroxydopamine, saralasin and hemorrhage on renal hemodynamics. J. Clin. Pharmacol. 1977; 17: 5-12.
55. Neiberger RE, Passmore J. Effects of dopamine on canine intrarenal blood flow distribution during hemorrhage. Kid. Int. 1979; 15: 219-226.
56. Jaschke W, Sievers RS, Lipton MJ, Cogan MG. Cine-computed tomographic assessment of regional renal blood flow. Acta Radiol. 1990; 31: 77-81.
57. Kompanowska-Jezierska E, Walkowska A, Johns EJ, Sadowski J. Early effects of renal denervation in the anaesthetised rat: natriuresis and increased cortical blood flow. J. Physiol. 2001; 531: 527-534.
58. Evans RG, Madden AC, Denton KM. Diversity of responses of renal cortical and medullary blood flow to vasoconstrictors in conscious rabbits. Acta Physiol. Scand. 2000; 169: 297-308.
59. Navakatikyan MA, Leonard BL, Evans RG, Malpas SC. Modelling the neural control of intrarenal blood flow. Clin. Exp. Pharmacol. Physiol. 2000; 27: 650-652.
60. Zucker IH, Wang W, Brandle M, Schultz HD, Patel KP. Neural regulation of sympathetic nerve activity in heart failure. Prog. Cardiovasc. Dis. 1995; 37: 397-414.
61. Rajapakse NW, Oliver JJ, Evans RG. Nitric oxide in responses of regional kidney blood flow to vasoactive agents in anesthetized rabbits. J. Cardiovasc. Pharmacol. 2002; 40: 210-219.
62. Eppel GA, Denton KM, Malpas SC, Evans RG. Nitric oxide in responses of regional kidney perfusion renal nerve stimulation and renal ischaemia. Pflügers Arch. 2003; 447: 205-213.
63. Guild S-J, Barrett CJ, Evans RG, Malpas SC. Interactions between neural and hormonal mediators of renal vascular tone in anaesthetized rabbits. Exp. Physiol. 2003; 88: 229-241.
64. Oliver JJ, Rajapakse NW, Evans RG. Effects of indomethacin on responses of regional kidney perfusion to vasoactive agents in rabbits. Clin. Exp. Pharmacol. Physiol. 2002; 29: 873-879.
65. Duke LM, Eppel GA, Widdop RE, Evans RG. Disparate roles of AT2-receptors in the renal cortical and medullary circulations of anesthetized rabbits. Hypertension 2003; 42: 200-205.
66. Denton KM, Finkelstein L, Flower RL, Shweta A, Evans RG, Anderson WP. Constriction of juxtamedullary arterioles in response to endothelin does not decrease medullary blood flow. FASEB J. 2003; 17 (5 part II): 587.5 (Abstract).
67. Correia AG, Denton KM, Evans RG. Effects of activation of vasopressin V1-receptors on regional kidney blood flow and glomerular arteriole diameters. J. Hypertens. 2001; 19: 649-657.
68. Lee LL, Evans RG, Eppel GA. Roles of α-adrenoceptor subtypes in regional renal vascular responses to renal nerve stimulation in rabbits. Clin. Exp. Pharmacol. Physiol. 2003; 30: A66 (Abstract).
69. Zou A-P, Cowley AWJr. α2-Adrenergic receptor-mediated increase in NO production buffers renal medullary vasoconstriction. Am. J. Physiol. 2000; 279: R769-R777.
70. Rajapakse NW, Eppel GA, Denton KM, Malpas SC, Evans RG. Do nitric oxide and prostaglandins protect the renal medullary circulation from ischemia during renal nerve stimulation? FASEB J. 2003. 17 (5 part II): 587.22 (Abstract).
71. Zimmerman BG. Actions of angiotensin on adrenergic nerve endings. Fed. Proc. 1978; 37: 199-202.
72. Bankir L, Bouby N, Trinh-Trang-Tan M-M. Heterogeneity of nephron anatomy. Kid. Int. 1987; 31(suppl. 20): S-25-S-39.
73. Beeuwkes R, Bonventre JV. Tubular organization and vascular-tubular relations in the dog kidney. Am. J. Physiol. 1975; 229: 695-713.
74. Vander A. Renal Physiology. 1995, New York: McGraw Hill.
75. Lübbers DW, Baumgärtl H. Heterogeneities and profiles of oxygen pressure in brain and kidney as examples of the pO2 distribution in the living tissue. Kid. Int. 1997; 51: 372-380.