Mechanosensitive (MS) ion channels are found in all types of living cells where they play an important role in mechanosensory transduction processes ranging from turgor control in bacteria and plant cells to hearing, touch, renal tubular function and blood pressure regulation in mammals. They convert mechanical stimuli acting upon membranes of biological cells into electrical or chemical signals (Hamill & Martinac, 2001). In the evolution of different life forms on Earth these ion channels may be among the oldest sensory transduction molecules that evolved as primary signalling elements in response to stimuli from the surrounding environment. The concept of ion channels gated by mechanical stimuli arose originally from studies of specialized mechanosensory neurons (Hamill & Martinac, 2001). Their discovery in embryonic chick skeletal muscle (Guharay & Sachs, 1984) and in frog muscle (Brehm et al., 1984) over twenty five years ago demonstrated the existence of MS ion channels in many non-specialized types of cells. (Sachs, 1988) Instrumental for the discovery of MS channels was the invention of the patch clamp technique (Hamill et al., 1981), which allowed the first direct measurements of single MS channel currents in a variety of non-specialized cells (Hamill & Martinac, 2001), including bacteria and archaea (Martinac, 2004). Studies of MS ion channels carried out over the last twenty five years have greatly contributed to our understanding of the molecular mechanisms underlying the physiology of mechanosensory transduction.

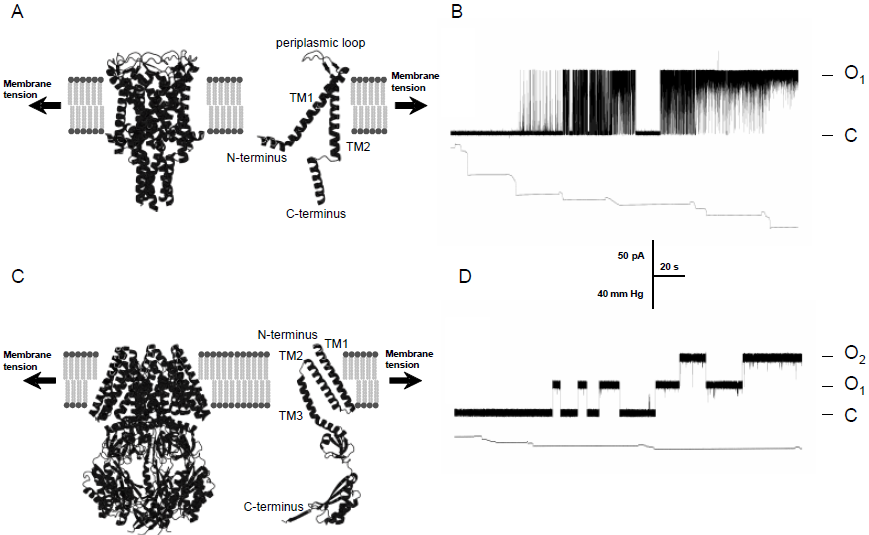
The cloning and structural determination of bacterial MscL and MscS channels (Figure), cloning and genetic analysis of the mec genes in Cenorhabditis elegans, genetic and functional studies of the TRP-type MS channels as well as functional and genetic studies of the TREK and TRAAK 2P-type K+ MS ion channels continue to promote our understanding of the role that MS channels play in the physiology of mechanosensory transduction in living organisms (Venkatachalam & Montell, 2007). In recent years the scientific and medical community has become increasingly aware of the importance of aberrant mechanosensitive channels contributing to pathophysiology of various diseases including heart failure and dysfunction, muscular dystrophy and polycystic kidney disease, to name a few (Venkatachalam & Montell, 2007; Martinac et al., 2008). At present, MS channel proteins are at the focus of structural, spectroscopic, computational and functional studies aiming to understand the molecular basis of mechanosensory transduction in living cells.
Brehm, P, Kullberg, R & Moody-Corbett, F. (1984) Journal of Physiology 350: 631-648.
Guharay, F & Sachs, F. (1984) Journal of Physiology 352: 685-701.
Hamill, OP & Martinac, B. (2001) Physiological Reviews 81:685-740.
Hamill, OP, Marty, A, Neher, E, Sakmann, B & Sigworth, FJ. (1981) Pflügers Archiv European Journal of Physiology 391: 85-100.
Martinac, B. (2004) Journal of Cell Science 117: 2449-2460.
Martinac, B, Saimi, Y & Kung, C. (2008) Physiological Reviews 88: 1449-1490.
Sachs, F. (1988) Critical Reviews in Biomedical Engineering 16: 141-169.
Venkatachalam, K & Montell, C.(2007) Annual Review of Biochemistry 76: 387-417.