
Contents




|
Programme
Contents 


|
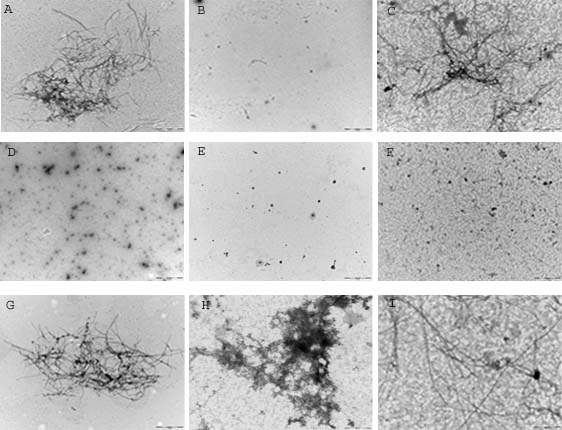
Accumulation of toxic amyloid-β in the cerebral cortex and hippocampus is a major pathological feature of Alzheimers disease (AD). The p75 neurotrophin receptor (p75NTR) is the common receptor for neurotrophins which can mediate cell death and neurite degeneration. Previous studies showed that the expression of p75NTR is increased in AD brain and activation of p75NTR by Aβ and nerve growth factor (NGF) does not always promote neuronal death but can promote survival of human neurons (Zhang et al., 2003). In this study, we investigated the effect of p75NTR extracellular domain fused with Fc fragment of human IgG (p75/Fc) on the Aβ assembly using transmission electron microscopy (TEM). Aβ peptide purchased from American Peptide (Sunnyvale,CA) was dissolved in DMEM at the final concentration of 22μmol/l. Samples were divided into 4 experimental groups and incubated at 4°C and 37°C respectively for 24 hours: 1, Aβ prepared immediately after dissolution without incubation; 2, Aβ incubated alone; 3, Aβ incubated with p75NTR (molar ratio 1:0.5) and 4, Aβ incubated with human IgG (molar ratio 1:0.5).
Zhang Y, Hong Y, Bounhar Y, Blacker M, Roucou X, Tounekti O, Vereker E, Bowers WJ, Federoff HJ, Goodyer CG, LeBlanc A. (2003) p75 neurotrophin receptor protects primary cultures of human neurons against extracellular amyloid beta peptide cytotoxicity, Journal of Neuroscience 23: 7385-7394.