Contents




|
Programme
Contents 


|
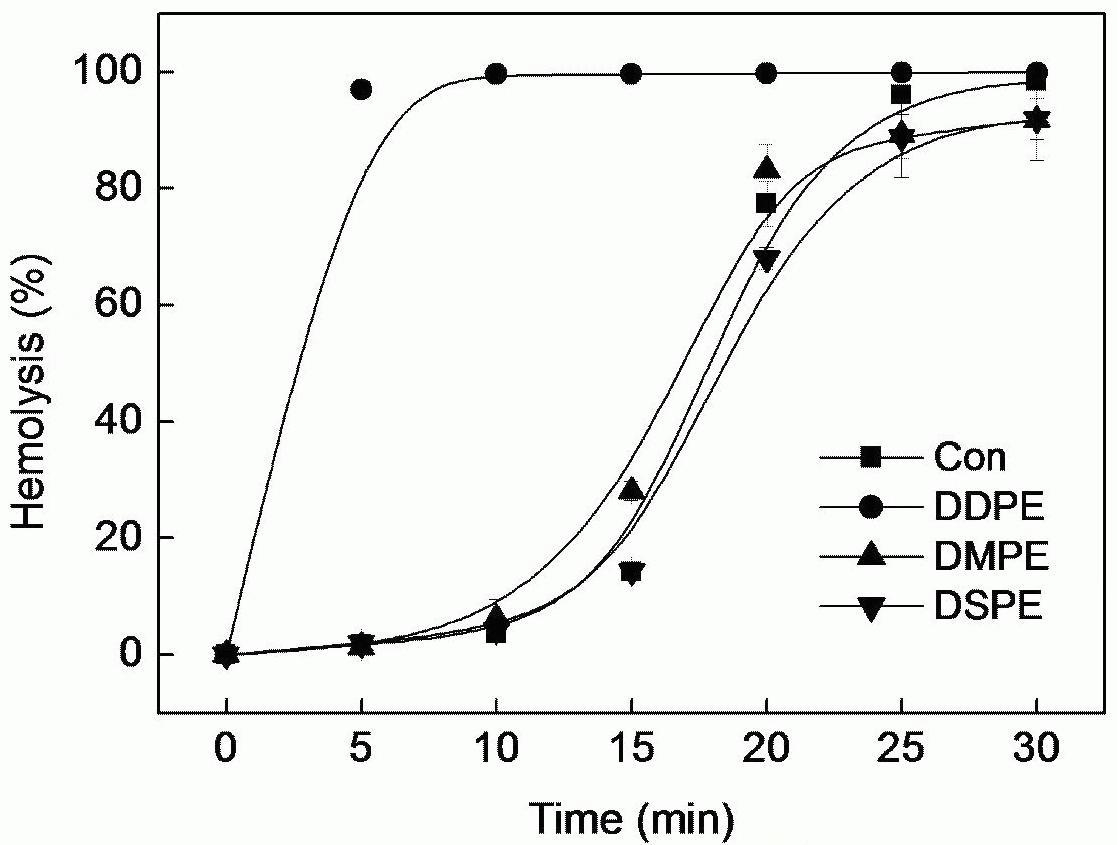
Tolaasin is a pore-forming antimicrobial peptide produced by Pseudomonas tolaasii and causes a brown blotch disease by disrupting membrane structures of cultivated mushrooms. It consists of 18 amino acids, its molecular mass is 1,985 Da and it has been demonstrated to form a left-handed α-helix. The mechanism of membrane-pore formation of tolaasin molecule has not known in detail. Since it forms enough for 4 turns of helix in solution, the length of tolaasin corresponds to near 20 Å, a little shorter than the thickness of membrane. Tolaasin channels are unstable in the artificial lipid bilayer and this may be explained by the comparison between the length of tolaasin channel and the thickness of lipid bilayer membrane. In control condition, bilayer was made with phosphatidyl ethanolamime (PE18:1/16:0) and phosphatidyl serine (PS18:1/18:0). The membrane is thicker than the estimated length of tolaasin channel and mismatch in thickness may make the channel unstable. Therefore, tolaasin may require phospholipids made with short length fatty acids to make the membrane thinner. In order to test this idea, the effects of various phospholipids composed of different length of fatty acids were investigated on haemolysis. When phosphatidyl ethanolamines made with decanoic acids (DDPE), myristic acids (DMPE), and stearic acids (DSPE) were added to the buffer containing RBCs and tolaasins, DDPE (200 nM) facilitates tolaasin-induced haemolysis. When the concentration of DDPE was adjusted from 0.2-200 nM, the haemolysis was stimulated at the concentrations above 2 nM, while fast, but not completed, hemolysis occurred only at 10 and 20 nM. The preincubated tolaasin and DDPE inhibited tolaasin-induced haemolysis. Binding of tolaasin and DDPE was completed within 5 minutes. Therefore, tolaasin molecules make more stable channels with phospholipids made with short length fatty acids.