There is growing evidence that reactive oxygen species play an important role in the development of skeletal muscle fatigue. As reducing agents that specifically target protein thiol groups (e.g. dithiothrietol) also slow muscle fatigue, reversible oxidation of protein thiol groups located on intracellular proteins involved in excitation-contraction coupling during fatigue has been hypothesized to be a primary mechanism contributing to fatigue in mammalian skeletal muscle (Ferreira & Reid, 2008). In this study, our aim was to provide evidence for this hypothesis by measuring whether consecutive bouts of muscle fatigue and post-fatigue recovery were associated with corresponding predicted changes in protein thiol oxidation levels.
Male Wistar (Rattus norvegicus) rats were anesthetized with an intraperitoneal injection of pentobarbital (65 mg/kg of body weight), and the extensor digitorum longus (EDL) muscles were carefully excised. EDL muscles were attached to a force transducer system and maintained in Krebs mammalian Ringer solution gassed with 5% CO2 and 95% O2 and maintained at 25°C. EDL muscles were subjected to two fatiguing stimulation runs which consisted of 10 min of sub-maximal tetanic contractions stimulated at 50 Hz for a duration of 500 ms each which was repeated every 15s. The two fatigue runs were separated by 60 min rest period. EDL muscles were frozen using aluminium clamps pre-cooled in liquid nitrogen before the first stimulation (unstimulated, n=6), after the first fatiguing stimulation (n=6), after the 60 min recovery (n=6) and after the second fatiguing stimulation (n=6). Protein thiol oxidation levels of the different conditions were measured and compared to that of unstimulated muscles. Protein thiol oxidation levels were determined using a fluorescent two-tag labelling technique involving the sequential labelling of reduced and oxidized protein thiol groups using two separate fluorescent tags on the same protein sample (Armstrong et al., 2011). There was no significant difference in protein thiol oxidation levels between unstimulated muscles (7.5 ± 0.3%, n=6) and muscles frozen immediately after dissection (7.6% ± 0.2%, n=6), indicating that bubbling the bath solution with 95% O2 did not influence protein thiol oxidation.
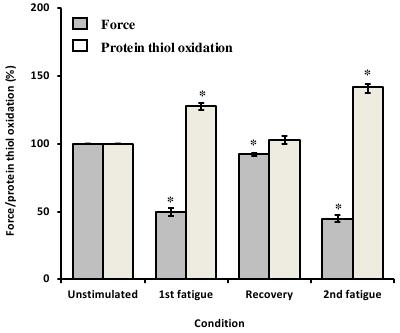
In response to the first fatiguing stimulation, muscle force decreased by 50 ± 3% and the mean protein thiol oxidation of fatigued muscles was 28 ± 3% (P<0.05) higher than unstimulated muscles (Figure 1). Following 60 min of rest, muscle force recovered to 93 ± 1% (P<0.05) of initial pre-fatigue force and mean protein thiol oxidation recovered to levels comparable to unstimulated muscles (Figure 1). After the second fatiguing stimulation, muscle force decreased by 45 ± 3%, a similar amount to the first fatigue run and the mean protein thiol oxidation levels of the muscles were 42 ± 6% (P<0.05) higher than that measured in unstimulated muscles (Figure 1).

In this study, we found that increased muscle fatigue was associated with increased muscle protein thiol oxidation, and the changes in force and protein thiol oxidation levels were consistent over two separate bouts of fatigue. Post-fatigue force recovery was associated with a decrease in muscle protein thiol oxidation levels, while non-thiol protein oxidation levels did not decrease during post-fatigue force recovery. These results are consistent with the hypothesis that muscle fatigue is mediated, at least in part, by an increase in muscle protein thiol oxidation.
Armstrong, A.E., Zerbes, R., Fournier, P.A., Arthur, P.G. (2011) A fluorescent dual labeling technique for the quantitative measurement of reduced and oxidized protein thiols in tissue samples. Free Radical Biology & Medicine 50(4), 510-517.
Ferreira, L.F., Reid, M.B. (2008) Muscle-derived ROS and thiol regulation in muscle fatigue. Journal of Applied Physiology 104, 853-860.