Engineered skeletal muscle has the potential to be a powerful model for studying muscle physiology, metabolism and function. It offers a number of significant advantages over traditional 2D cell culture including significantly increased lifespan, more physiologically relevant morphology and measurement of functional performance. Therefore we established a method for routinely producing engineered (3D) muscle tissue from the commonly-used C2C12 cell line and compared it with traditional 2D cell culture of the same cells using histochemistry and microscopy.
Methods: The protocol was essentially as described elsewhere (Khodabukus & Baar, 2009). Small (35 mm) culture dishes were coated in Sylgard and left to cure for a week. Two anchors were created using suture silk pinned down by insect pins (held in place by the Sylgard). The engineered tissue would form between these two anchors. A scaffold pro-gel was made by combining thrombin, aprotinin and genipin in DMEM, and was pipetted onto the pre-prepared culture dishes. Fibrinogen was added to initiate the formation of the gel. The culture dishes were then placed in an incubator for one hour to allow the gel to set, after which the dishes were seeded with C2C12 cells. From this point the tissue constructs took two weeks to mature, after which they could be used for functional testing. However, as traditional C2C12 cultures typically survive for only 7-10 days after differentiation, we used histochemistry to assess the morphology of the 3D constructs and compare them with traditional 2D cell culture with respect to developmental maturity and cytoskeletal organisation after just seven days.

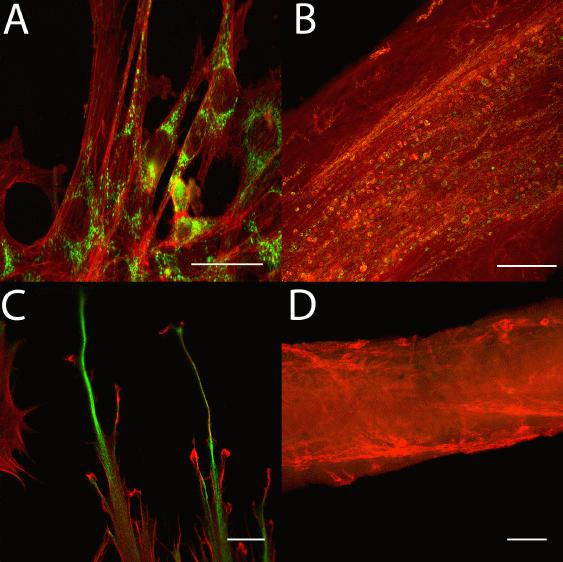
Results: In our hands, this protocol allowed robust production of engineered muscle tissue from C2C12 cells. We have produced 62 such constructs, with a lifespan of up to 48 days, in approximately a six month period. Histochemistry revealed profound differences in cell morphology between C2C12 cells dependent on 3D (Figure panels B & D) vs 2D (Panels A & C) culture conditions. Immunofluorescent staining of the developmental intermediate filament vimentin (green in Panels C & D) was more prominent on 2D cultured muscle cells (Panel C) than the engineered constructs (Panel D) suggesting a greater degree of cell maturity in the tissue constructs. Phalloidin staining of F-actin (red) showed that the constructs were beginning to organize themselves in parallel fibre-like structures after just one week (Panel B), while the cells in 2D culture lacked any sort of organisation when observed under identical conditions (Panel A). Immunostaining of cytochrome C (green in Panels A & B) showed that in the 3D constructs, mitochondria were located along the fibre-like structures (Panel B), whereas in 2D culture the mitochondria were located predominantly around the nucleus (Panel A). (Scale bars: A 25μm; B 75μm; C 10μm; D 100μm)
Conclusions: These preliminary results suggest that even after seven days, muscle tissue engineered from C2C12 cells was more morphologically mature and physiologically relevant than traditional 2D culture of C2C12 cells. Further work is required to characterize the tissue constructs' metabolic and contractile characteristics.
Khodabukus A, Baar K. (2009) Tissue Engineering Part C 15(3): 501-11.