1. With the onset of dynamic whole body exercise, contraction-induced mechanical and biochemical stimuli within locomotor muscle cause an increase in the discharge frequency of thinly myelinated (group III) and unmyelinated (group IV) nerve fibres located within the muscle.
2. These thin fibre muscle afferents project to various sites within the central nervous system and thereby substantially influence the exercising human.
3. First, group III/IV muscle afferents are the afferent arm of cardiovascular and ventilatory reflex responses which are mediated in the nucleus tractus solitarii and the ventrolateral medulla. Neural feedback from working skeletal muscle is therefore a vital component in providing a high capacity for endurance exercise since muscle perfusion and O2 delivery determine the fatigability of skeletal muscle.
4. Second, group III/IV muscle afferents facilitate “central fatigue” (failure, or unwillingness, of the central nervous system to “drive” motoneurons) by exerting inhibitory influences on central motor drive during exercise.
5. Taken together, group III/IV muscle afferents play a substantial role in a human’s susceptibility to fatigue and capacity for endurance exercise.
With the onset of exercise, contraction-induced mechanical and chemical stimuli begin to activate molecular receptors located on the terminal end of both thinly myelinated (group III) and unmyelinated (group IV) nerve fibres with their receptive fields within skeletal muscle. The exercise-induced activation of these receptors increases the spontaneous discharge of the thin fibre muscle afferents1-4 which project, via the lumbar dorsal horn of the spinal cord,5,6 to various sites within the CNS, many of which are currently unknown. The central projection of group III and IV muscle afferents plays a major role for the exercising human.7-9 The purpose of this brief report is to emphasize the significance of the central effects of these thin fibre muscle afferents on a) the cardiovascular and ventilatory responses, and b) on the regulation of central motor drive during high intensity endurance exercise.
Ventilatory and cardiovascular responses to exercise are primarily regulated by two largely separate systems. The first, a feedforward mechanism, termed “central command”, elicits cardiovascular and ventilatory responses to exercise.10 The second, a feedback mechanism, reflexly changes ventilation and circulation as a consequence of limb muscle contraction.7,11 The focus of this paper is on the later.
The first experimental evidence supporting the hypothesis that a reflex response from working muscle might account for a proportion of the cardiovascular12 and ventilatory13 response to exercise in humans was published about 70 years ago. Ever since, numerous animal and human studies have confirmed a key role for muscle afferents in evoking these responses during exercise.7,11,14 Specifically, the above mentioned non-nociceptive group III/IV muscle afferents, the so-called “ergoreceptors”,3,15,16 depict the afferent arm of the cardiovascular and ventilatory reflexes7,17-20 which are mediated via neural circuits in the nucleus tractus solitarii and the ventrolateral medulla.21
It is still controversial whether afferent feedback from exercising muscle has a considerable contribution to the cardioventilatory response during whole body endurance exercise or whether the functional significance of this input is limited to conditions of muscle ischemia as produced in sustained isometric contractions with compromised blood flow.22 In other words, it remains uncertain whether continuous muscle afferent feedback is necessary for adequate ventilatory and circulatory responses during normal rhythmic endurance exercise; or whether these responses are primarily determined by central command10 and muscle afferents only depict a temporary error signal23 to the brainstem reporting an acute mismatch between blood/oxygen supply and demand.18
Recent human studies using local anaesthetics (lumbar epidural space) to block the central projection of group III/IV muscle afferents during whole body endurance exercise (leg cycling) found attenuated, similar, or even increased cardiovascular and ventilatory responses when the identical exercise was performed with blocked muscle afferents.24-29 Although some of these studies conform to the idea that continuous afferent feedback is necessary for adequate ventilatory and circulatory responses, others contradict leaving the exact role of muscle afferents in the cardioventilatory control during endurance exercise controversial. At least a part of these conflicting findings might be explained by the use of local epidural anaesthetics which attenuate efferent as well as afferent nerve activity. The effects of local anaesthetics on efferent nerves cause a drug induced “muscle weakening”30 which inevitably requires an increase in central motor drive in order to work at / maintain a given external workload. Thus, afferent blockade using local anaesthetics creates a condition of reduced feedback in the face of increased feedforward and this increase in central command, by itself, augments the cardioventilatory response to exercise as demonstrated in curarization experiments.31-33 Therefore, the results from previous studies using local epidural anaesthetics necessitate careful interpretation since the resultant net effect on ventilatory and circulatory responses during exercise with blocked afferent feedback depends upon the degree to which the increase in central motor drive balances the reduced feedback from the working limb muscle.28,30

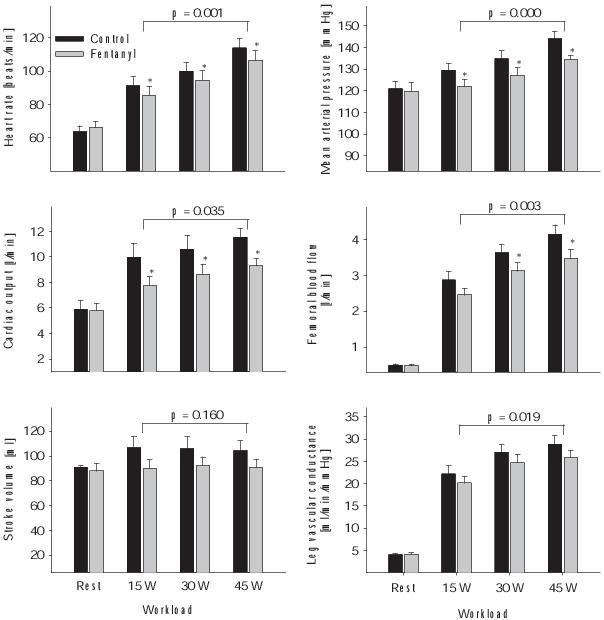
Figure 1. Circulatory responses at rest and during the final minute of one leg knee-extension exercise. The exercise was performed at a low, middle, and high exercise intensity (3 min each) with (black bars) and without (grey bars) the central projection of lower limb group III/IV muscle afferents. The P-value indicates the overall main effect of fentanyl. *P<0.05. n = 9. Modified from Amann et al. (2011)35
Two recent studies designed to circumvent the confounding impact of local epidural anaesthetics provide valuable insights into the effects of muscle afferents on the circulatory and ventilatory response to whole body endurance exercise.34,35 By using lumbar intrathecal fentanyl, a selective μ-opioid receptor agonist, we were able to inhibit the central projection of group III/IV muscle afferents without affecting the muscle's force generating capacity and therefore without affecting central motor drive / feedforward during the exercise. The outcome of these studies clearly shows that when group III/IV muscle afferents from the lower limbs are blocked during endurance exercise of various intensities ranging from mild to heavy, circulation (Figure 1) and pulmonary ventilation (Figure 2) are substantially compromised. This not only causes arterial hypoxemia, attenuates perfusion pressure and blood flow which eventually reduces O2 delivery to the working muscles, but also facilitates ventilatory and metabolic acidosis34,35 – all of which combine to accelerate the development of peripheral locomotor muscle fatigue during exercise.36 These findings suggest that continuous sensory feedback from working skeletal muscle might depict a vital component in providing a high capacity for rhythmic endurance exercise since controlled muscle perfusion and O2 delivery determine the fatigability of skeletal muscle and thus affect its performance.36-38
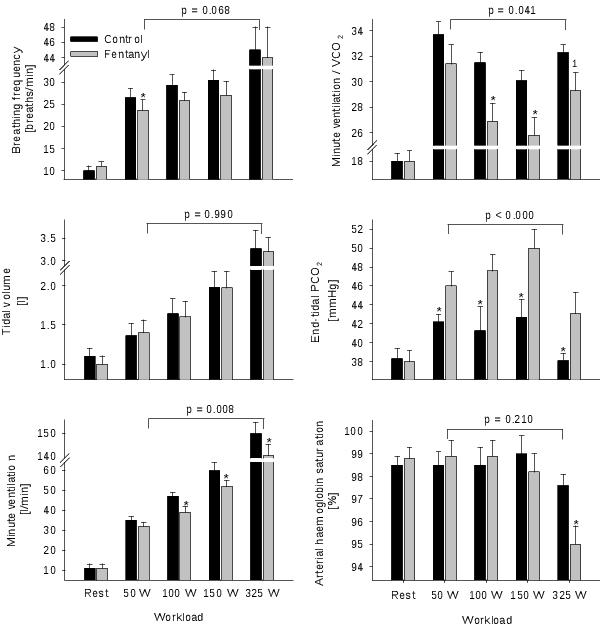
Figure 2. Ventilatory response and haemoglobin saturation at rest and during the final minute of bicycle exercise. The exercise was performed at 4 different workloads (3 min each) with (black bars) and without (grey bars) the central projection of lower limb group III/IV muscle afferents. The P-value indicates the overall main effect of fentanyl. *P<0.05. 1P=0.08. n = 7. From Amann et al. (2010).34
Group III/IV muscle afferents are also known to facilitate central fatigue (i.e., the CNS-mediated reduction in “driving” motoneurons39) by providing inhibitory feedback to the regulation of central motor drive and voluntary muscle activation during exercise.8 This was initially shown during maximal isometric exercise of a single muscle (for review see Gandevia, 20018). For example, when the discharge rate, and thus the central projection of group III/IV muscle afferents is maintained following a 2-min maximal voluntary biceps brachii contraction (via arresting blood flow to and from the arm), central motor drive and voluntary muscle activation remain low and do not recover until circulation is restored and the firing frequency of group III/IV afferents recovers.40
The inhibitory effect of group III/IV muscle afferents on the regulation of the magnitude of central motor drive is also of critical relevance during high-intensity whole body endurance exercise.9 For example, when subjects performed a 5 km cycling time trial with blocked group III/IV muscle afferents (via lumbar intrathecal fentanyl), the centrally-mediated inhibitory effect of these ergoreceptors was “attenuated” and central motor drive was less restricted and significantly higher as compared to the identical time trial performed with a placebo (Figure 3).41 The higher central motor drive resulted in a substantially higher power output during the first half of the race and the CNS “tolerated” the development of peripheral locomotor muscle fatigue substantially beyond levels observed following the placebo time trial (i.e. performed with intact afferent feedback).41
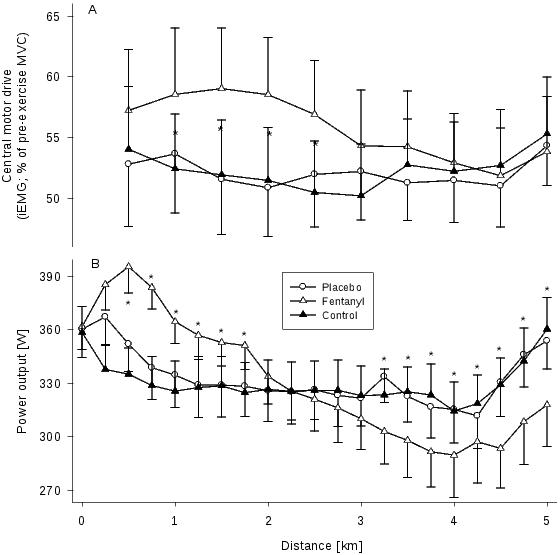
Figure 3. Effect of lower limb muscle afferent feedback on central motor drive (CMD) and power output. Data were obtained during 5 km cycling time trials performed under control conditions, with a placebo injection (interspinous ligament injection of sterile normal saline, L3-L4), and with intrathecal fentanyl (L3-L4). A: Effects of blocking the central projection of group III/IV muscle afferents on CMD (estimated via changes in integrated vastus lateralis EMG (iEMG)) during the race. Mean vastus lateralis iEMG was normalized to the iEMG obtained from pre-exercise quadriceps MVC maneuvers. Each point represents the mean CMD of the preceding 0.5 km section. B: Mean power output during the 5 km time trial with and without impaired afferent feedback. The subjects were required to reach an individual target power output before the race was started. *P<0.05 (Fentanyl vs Placebo). n = 9. From Amann et al. (2009).41
Based on sufficient correlative and experimental evidence from us and others over the past years, we propose that the CNS processes neural feedback from locomotor muscle afferents and regulates exercise by adjusting central motor drive in order to confine the development of locomotor muscle fatigue during high intensity endurance performance (i.e. cycling exercise) to a critical threshold, beyond which the level of associated sensory input would not be tolerated.41-45 In other words, peripheral locomotor muscle fatigue and associated intramuscular metabolic changes exert, via the effects on lower limb muscle afferent feedback, an inhibitory influence on the regulation of central motor drive and thereby limit the development of peripheral fatigue to an individual threshold. This centrally mediated restriction in the development of peripheral locomotor muscle fatigue might help to prevent excessive disturbance of muscle homeostasis and potential harm to the organism.46
Although some information is available regarding the brain areas nociceptive muscle afferents are projecting to47,48 the exact anatomical sites within the CNS which mediate the effects of non-nociceptive group III/IV muscle afferents on central motor drive are unknown. Neural circuits involved in generating motor cortical output "upstream" from the motor cortex,49,50 as well as the motor cortex itself,51 have been suggested.
Group III and IV muscle afferents play a pivotal twofold role for the endurance exercising human. First, continuous afferent feedback from working locomotor muscle is essential for evoking appropriate ventilatory and circulatory responses during exercise – both of which are critical prerequisites for preventing premature fatigue and accomplishing an optimal performance. And second, group III/IV muscle afferents provide inhibitory feedback to the CNS and thereby influence the regulation of central motor drive and limit the development of peripheral fatigue to a critical threshold, presumably to protect the organism from excessive exhaustion and potentially harm.
This work was supported by the US National Heart, Lung, and Blood Institute (HL-103786).
1. Adreani CM, Hill JM, Kaufman MP. Responses of group III and IV muscle afferents to dynamic exercise. J. Appl. Physiol. 1997, 82: 1811-1817.
2. Pickar JG, Hill JM, Kaufman MP. Dynamic exercise stimulates group III muscle afferents. J. Neurophysiol. 1994, 71: 753-760.
3. Light AR, Hughen RW, Zhang J, Rainier J, Liu Z, Lee J. Dorsal root ganglion neurons innervating skeletal muscle respond to physiological combinations of protons, ATP, and lactate mediated by ASIC, P2X, and TRPV1. J. Neurophysiol. 2008, 100: 1184-1201.
4. Kaufman MP, Hayes SG, Adreani CM, Pickar JG. Discharge properties of group III and IV muscle afferents. Adv. Exp. Med. Biol. 2002, 508: 25-32.
5. Wilson LB, Hand GA. The pressor reflex evoked by static contraction: neurochemistry at the site of the first synapse. Brain Res. Brain Res. Rev. 1997, 23: 196-209.
6. Wilson LB, Andrew D, Craig AD. Activation of spinobulbar lamina I neurons by static muscle contraction. J. Neurophysiol. 2002, 87: 1641-1645.
7. Kaufman MP, Forster HV. Reflexes controlling circulatory, ventilatory and airway responses to exercise. In: Handbook of Physiology Section 12: Exercise: Regulation and Integration of Multiple Systems, edited by Rowell LB, and Shepherd JT. New York: Oxford University Press, 1996, p. 381-447.
8. Gandevia SC. Spinal and supraspinal factors in human muscle fatigue. Physiol. Rev. 2001, 81: 1725-1789.
9. Amann M. Central and peripheral fatigue: interaction during cycling exercise in humans. Med. Sci. Sports Exerc. 2011, 43: 2039-2045.
10. Waldrop TG, Eldridge FL, Iwamoto GA, Mitchell JH. Central neural control of respiration and circulation during exercise. In: Handbook of Physiology Section 12: Exercise: Regulation and Integration of Multiple Systems, edited by Rowell LB, and Shepherd JT. New York: Oxford University Press, 1996, p. 333-380.
11. Secher NH, Amann M. Human investigations into the exercise pressor reflex. Exp. Physiol. 2012, 97: 59-69.
12. Alam M, Smirk FH. Observations in man upon a blood pressure raising reflex arising from the voluntary muscles. J. Physiol. 1937, 89: 372-383.
13. Asmussen E, Nielsen M, Wieth-Pedersen G. Cortical or reflex control of respiration during muscular work. Acta Physiol. Scand. 1943, 6: 168-175.
14. Smith SA, Mitchell JH, Garry MG. The mammalian exercise pressor reflex in health and disease. Exp. Physiol. 2006, 91: 89-102.
15. Kniffeki KD, Mense S, Schmidt RF. Muscle receptors with fine afferent fibers which may evoke circulatory reflexes. Circ. Res. 1981, 48: I25-31.
16. Light AR, White AT, Hughen RW, Light KC. Moderate exercise increases expression for sensory, adrenergic, and immune genes in chronic fatigue syndrome patients but not in normal subjects. J. Pain 2009, 10: 1099-1112.
17. McCloskey DI, Mitchell JH. Reflex cardiovascular and respiratory responses originating in exercising muscle. J. Physiol. 1972, 224: 173-186.
18. Mitchell JH, Kaufman MP, Iwamoto GA. The exercise pressor reflex: its cardiovascular effects, afferent mechanisms, and central pathways. Ann. Rev. Physiol. 1983, 45: 229-242.
19. Boushel R. Muscle metaboreflex control of the circulation during exercise. Acta Physiol. (Oxford) 2010, 199: 367-383.
20. Coote JH, Hilton SM, Perez-Gonzalez JF. The reflex nature of the pressor response to muscular exercise. J. Physiol. 1971, 215: 789-804.
21. Craig AD. Distribution of brainstem projections from spinal lamina I neurons in the cat and the monkey. J. Comp. Neurol. 1995, 361: 225-248.
22. Rowell LB, O'Leary DS. Reflex control of the circulation during exercise: chemoreflexes and mechanoreflexes. J. Appl. Physiol. 1990, 69: 407-418.
23. O'Leary DS, Sheriff DD. Is the muscle metaboreflex important in control of blood flow to ischemic active skeletal muscle in dogs? Am. J. Physiol. Heart Circ. 1995, 268: H980-986.
24. Freund PR, Rowell LB, Murphy TM, Hobbs SF, Butler SH. Blockade of the pressor response to muscle ischemia by sensory nerve block in man. Am. J. Physiol. Heart Circ. 1979, 237: H433-439.
25. Kjaer M, Hanel B, Worm L, Perko G, Lewis SF, Sahlin K, Galbo H, Secher NH. Cardiovascular and neuroendocrine responses to exercise in hypoxia during impaired neural feedback from muscle. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1999, 277: R76-85.
26. Friedman DB, Brennum J, Sztuk F, Hansen OB, Clifford PS, Bach FW, Arendt-Nielsen L, Mitchell JH, and Secher NH. The effect of epidural anaesthesia with 1% lidocaine on the pressor response to dynamic exercise in man. J. Physiol. 1993, 470: 681-691.
27. Smith SA, Querry RG, Fadel PJ, Gallagher KM, Stromstad M, Ide K, Raven PB, and Secher NH. Partial blockade of skeletal muscle somatosensory afferents attenuates baroreflex resetting during exercise in humans. J. Physiol. 2003, 551: 1013-1021.
28. Fernandes A, Galbo H, Kjaer M, Mitchell JH, Secher NH, Thomas SN. Cardiovascular and ventilatory responses to dynamic exercise during epidural anaesthesia in man. J. Physiol. 1990, 420: 281-293.
29. Strange S, Secher NH, Pawelczyk JA, Karpakka J, Christensen NJ, Mitchell JH, and Saltin B. Neural control of cardiovascular responses and of ventilation during dynamic exercise in man. J. Physiol. 1993, 470: 693-704.
30. Amann M, Proctor LT, Sebranek JJ, Eldridge MW, Pegelow DF, Dempsey JA. Somatosensory feedback from the limbs exerts inhibitory influences on central neural drive during whole body endurance exercise. J. Appl. Physiol. 2008, 105: 1714-1724.
31. Ochwadt B, Bücherl E, Kreuzer H, Löschcke HH. Beeinflussung der Atemsteigerung bei Muskelarbeit durch partiellen neuromuskulären Block (Tubocurarine). Pflügers Arch. Gesamte Physiol. Menschen Tiere 1959, 269: 613-621.
32. Galbo H, Kjaer M, Secher NH. Cardiovascular, ventilatory and catecholamine responses to maximal dynamic exercise in partially curarized man. J. Physiol. 1987, 389: 557-568.
33. Asmussen E, Johansen SH, Jorgensen M, Nielsen M. On the nervous factors controlling respiration and circulation during exercise. Experiments with curarization. Acta Physiol. Scand. 1965, 63: 343-350.
34. Amann M, Blain GM, Proctor LT, Sebranek JJ, Pegelow DF, Dempsey JA. Group III and IV muscle afferents contribute to ventilatory and cardiovascular response to rhythmic exercise in humans. J. Appl. Physiol. 2010, 109: 966-976.
35. Amann M, Runnels S, Morgan DE, Trinity JD, Fjeldstad AS, Wray DW, Reese VR, Richardson RS. On the contribution of group III and IV muscle afferents to the circulatory response to rhythmic exercise in humans. J. Physiol. 2011, 589: 3855-3866.
36. Amann M, Blain GM, Proctor LT, Sebranek JJ, Pegelow DF, Dempsey JA. Implications of group III and IV muscle afferents for high intensity endurance exercise performance in humans. J. Physiol. 2011, 589: 5299-5309.
37. Hepple RT. The role of O2 supply in muscle fatigue. Can. J. Appl. Physiol. 2002, 27: 56-69.
38. Amann M, Calbet JA. Convective oxygen transport and fatigue. J. Appl. Physiol. 2008, 104: 861-870.
39. Bigland-Ritchie BR, Dawson NJ, Johansson RS, Lippold OC. Reflex origin for the slowing of motoneurone firing rates in fatigue of human voluntary contractions. J. Physiol. 1986, 379: 451-459.
40. Gandevia SC, Allen GM, Butler JE, Taylor JL. Supraspinal factors in human muscle fatigue: evidence for suboptimal output from the motor cortex. J. Physiol. 1996, 490: 529-536.
41. Amann M, Proctor LT, Sebranek JJ, Pegelow DF, Dempsey JA. Opioid-mediated muscle afferents inhibit central motor drive and limit peripheral muscle fatigue development in humans. J. Physiol. 2009, 587: 271-283.
42. Amann M, Eldridge MW, Lovering AT, Stickland MK, Pegelow DF, Dempsey JA. Arterial oxygenation influences central motor output and exercise performance via effects on peripheral locomotor muscle fatigue in humans. J. Physiol. 2006, 575: 937-952.
43. Romer LM, Haverkamp HC, Amann M, Lovering AT, Pegelow DF, Dempsey JA. Effect of acute severe hypoxia on peripheral fatigue and endurance capacity in healthy humans. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292: R598-606.
44. Amann M, Dempsey JA. Locomotor muscle fatigue modifies central motor drive in healthy humans and imposes a limitation to exercise performance. J. Physiol. 2008, 586: 161-173.
45. Amann M, Romer LM, Subudhi AW, Pegelow DF, Dempsey JA. Severity of arterial hypoxaemia affects the relative contributions of peripheral muscle fatigue to exercise performance in healthy humans. J. Physiol. 2007, 581: 389-403.
46. Noakes TD. Time to move beyond a brainless exercise physiology: the evidence for complex regulation of human exercise performance. Appl. Physiol. Nutr. Metab. 2011, 36: 23-35.
47. Brooks JC, Zambreanu L, Godinez A, Craig AD, Tracey I. Somatotopic organisation of the human insula to painful heat studied with high resolution functional imaging. NeuroImage 2005, 27: 201-209.
48. Craig AD. Pain mechanisms: labeled lines versus convergence in central processing. Ann. Rev. Neurosci. 2003, 26: 1-30.
49. Hilty L, Lutz K, Maurer K, Rodenkirch T, Spengler CM, Boutellier U, Jancke L, Amann M. Spinal opioid receptor-sensitive muscle afferents contribute to the fatigue-induced increase in intracortical inhibition in healthy humans. Exp. Physiol. 2011, 96: 505-517.
50. Taylor JL, Allen GM, Butler JE, Gandevia SC. Supraspinal fatigue during intermittent maximal voluntary contractions of the human elbow flexors. J. Appl. Physiol. 2000, 89: 305-313.
51. Sidhu SK, Bentley DJ, Carroll TJ. Locomotor exercise induces long-lasting impairments in the capacity of the human motor cortex to voluntarily activate knee extensor muscles. J. Appl. Physiol. 2009, 106: 556-565.