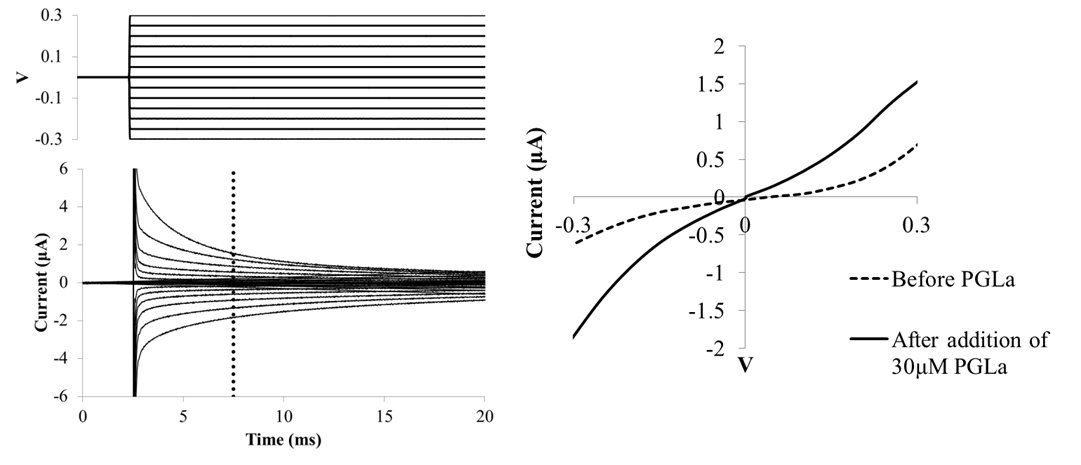
Bacterial resistance to conventional antibiotics is an increasing problem and the pursuit of alternative drug regimens is becoming more urgent. Compounds that target bacterial membranes are considered to be excellent drug candidates. The skin of the African clawed frog Xenopus laevis contains amphipathic peptides such as PGLa (Zasloff, 1987) which are cationic and have a much higher affinity for negatively charged bacterial membranes than for uncharged eukaryotic membranes (Glukhov et al., 2005). We describe new techniques to study the insertion of pore forming antimicrobial peptides (AMPs) into lipid bilayers using tethered bilayer lipid membranes (tBLMs). AC impedance spectroscopy using tBLMs confirmed that PGLa preferentially forms pores in membranes containing negatively charged POPG lipids. A dose response profile of PGLa insertion indicates that insertion is preferred only when a threshold concentration of the peptide is reached, supporting previous NMR data (Afonin et al., 2008). A novel tBLM approach was employed which permits the measurement of conductance in the presence of a transient DC potential across the membrane. A consequence of tethering a membrane to a gold surface is that electrical contact to the PBS bathing solution is intrinsically capacitive, preventing the direct application of a steady-state voltage across the bilayer. However, by using pulsed waveforms, defined potentials may be expressed across the membrane for tens to hundreds of milliseconds, and the resulting I-V plots provide valuable data about AMP insertion rates and voltage dependence (see figure).

Using this voltage clamp technique in the presence of PGLa, we demonstrated AMP insertion into zwitterionic and negatively charged lipid membranes can be rapidly measured and compared. To better understand the voltage dependence of peptide insertion into tBLMs, ramped potentials can also be applied which can quickly determine the potential thresholds of peptide insertion and pore formation. The advantages of using tBLMs with these electrophysiology techniques are:
Afonin S, Grage SL, Ieronimo M, Wadhwani P & Ulrich AS. (2008). Journal of the American Chemical Society 130, 16512-16514.
Glukhov E, Stark M, Burrows LL & Deber CM. (2005). Journal of Biological Chemistry 280, 33960-33967.
Zasloff M. (1987) Proceedings of the National Academy of Sciences 84, 5449-5453.