Glutamate transporters, or Excitatory Amino Acid Transporters (EAATs), are concentrative solute carriers that use steep gradients of Na+ to sequester glutamate and aspartate intracellularly to terminate the action of these neurotransmitters (Danbolt, 2001). Detailed structural information for the EAATs remains elusive, as X-ray crystallography on mammalian membrane-bound proteins remains difficult. For this reason, a homologous protein has been identified which provides structural insights into the transport mechanisms and kinetics of the EAATs.
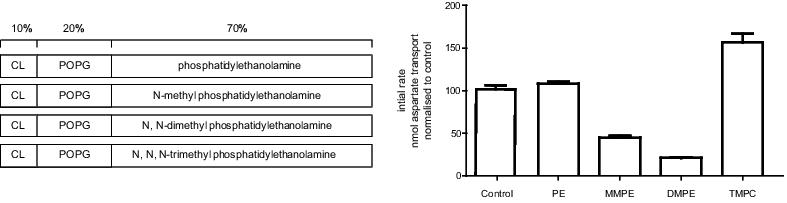
The Na+/aspartate symporter from Pyrococcus horikoshii, GltPh, has been crystallized in a variety of states representing static conformations of the transport cycle (Boudker et al., 2007). The aim of this study is to determine whether the activity of GltPh is influenced by the lipid bilayer. Purified GltPh was reconstituted into liposomes comprised of 10% cardiolipin, 20% phosphatidylglycerol and 70% of phosphatidylethanolamine or its mono-, di- or tri- methylated derivatives (see Figure). Radiolabelled 3H-L-aspartate uptake studies were conducted and the activity in these different lipid compositions compared to a well-documented control condition (Ryan et al., 2009).

The ability for GltPh transport rate to be affected without altering affinities for co-transported Na+ or substrate indicate that conformational changes in the transport cycle have been affected, with the lipid bilayer determining the favourability of conformational transitions.
Boudker O, Ryan RM, Yernool D, Shimamoto K, Gouaux E (2007) Coupling substrate and ion binding to extracellular gate of sodium-dependent aspartate transporter. Nature 445: 387-393.
Danbolt, NC (2001) Glutamate uptake. Progress in Neurobiology 65: 1-105.
Ryan RM, Compton EL, Mindell JA (2009) Functional characterization of a Na+-dependent aspartate transporter from Pyrococcus horikoshii. Journal of Biological Chemistry 284: 17540-17548.