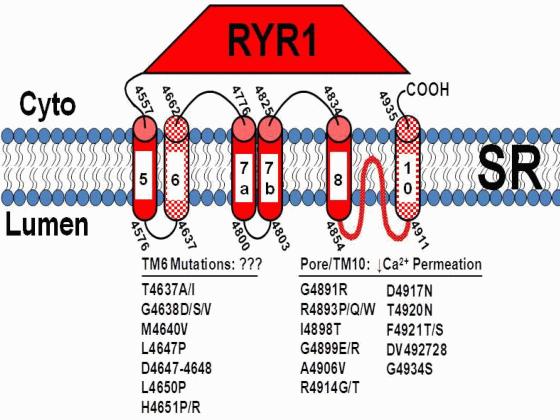
Mutations in the type I ryanodine receptor (RyR1) that result in malignant hyperthermia (MH) and central core disease (CCD) can be found throughout the RyR1 sequence. However, the vast majority of CCD mutations are located in the C-terminal portion of the protein, which contains the transmembrane and selectivity filter/pore-lining regions of the channel. Interestingly, a large number of the C-terminal CCD mutations are found in two clusters: one in the selectivity filter/pore-lining region and a second cluster within the conserved “M6” transmembrane domain (TM6; or M2 in the model of Takeshima et al., 1989; see the Figure) of the channel. The Dirksen laboratory has previously found that CCD mutations in the RyR1 selectivity filter result in a form of excitation-contraction (EC) uncoupling in which there is reduced calcium release in the absence of store depletion, due to a disruption in calcium permeation (Avila et al., 2003; Loy et al., 2011). The effects of the TM6 mutations on RyR1 function have not previously been assessed and are important in terms of the molecular changes leading to CCD and also in the elucidating role of TM6 in ion channel function.

We have assessed the structure of TM6 using structural models and circular dichroism (CD) of a synthetic TM6 peptide with the sequence of the TM6 region. The effect of 11 different CCD mutations in TM6 on EC coupling and bi-directional coupling between RyR1 and the dihydropyridine receptor (DHPR) was determined following expression of the mutant RyR1 protein in RyR1-null (dyspedic) myotubes as described in Avila et al. (2003). Conductance and gating of channels expressed in, and isolated from, HEK293 cells were measured following incorporation of the channels into planar lipid bilayers as described in Goonasekera et al. (2009).
An α-helical structure for the TM6 residues was predicted and indicated in CD spectra of the TM6 peptide embedded in liposome membrane. An EC uncoupling phenotype was observed for a subset of CCD mutations (L4647P, Delta4647-48, H4651P, H4651R) that were all located in the centre and on one face of the predicted TM6 α-helix. EC coupling and RyR1 function were essentially unaltered for CCD mutations located at either the N-terminal end (T4637A/I, G4638D/S/V, M4640V) or on the opposing face of the TM6 α-helix (L4650P). Immunocytochemistry and whole-cell patch clamp studies revealed that all four of the EC uncoupling mutants in TM6 properly co-localized with the DHPR within SR-sarcolemmal peripheral junctions and DHPR calcium currents were enhanced, indicating intact retrograde RyR1/DHPR coupling. Additional mutations (L4644P/R, L4647R, L4649P, A4653P, A4655P/R) were engineered to precisely define the boundaries and helix-sidedness of the EC uncoupling module in TM6. Bilayer studies showed that while potassium and calcium permeation were unaltered by the Delta4647-48, L4650P, and H4651P TM6 mutations, each of these mutants exhibited a markedly reduced average channel open probability due to a combination of a modest reduction in channel mean open time and a larger increase in channel mean closed time.
Together, these results demonstrate that one face of TM6 functions as an important RyR1 channel gating module and that mutations of residues within this domain result in EC uncoupling by reducing channel opening in the absence of a change in calcium ion permeation. It is likely that this effect on EC coupling and channel gating depends on the disruption of interactions between the EC uncoupling face of TM6 that we have identified and a corresponding face on one of the adjacent transmembrane sequences that determine the duration of channel closure.
Avila G, O'Connell KM, Dirksen RT. (2003) Journal of General Physiology 121: 277-86.
Loy RE, Orynbayev M, Xu L, Andronache Z, Apostol S, Zvaritch E, MacLennan DH, Meissner G, Melzer W, Dirksen RT. (2011) Journal of General Physiology 137: 43-57.
Takeshima H, Nishimura S, Matsumoto T, Ishida H, Kangawa K, Minamino N, Matsuo H, Ueda M, Hanaoka M, Hirose T, et al. (1989) Nature 339: 439-45.
Goonasekera SA, Beard NA, Groom L, Kimura T, Lyfenko AD, Rosenfeld A, Marty I, Dulhunty AF, Dirksen RT. (2007) Journal of General Physiology 130: 365-78.