Voltage gated sodium (Nav) channels are responsible for the function of excitable cells and they are therapeutic targets for some related disorders such as cardiac and neuropathic diseases. μ-Conotoxins are Nav channel blockers which have been well studied experimentally and recently suggested for treatment of chronic pain. Since 2011, crystal structures of prokaryotic Nav channels provide new data for exploring Nav channels. They also provide new templates for constructing homology models of eukaryotic Nav channels and studying their interactions with toxins. Here, we construct a new model of Nav1.4 and study the binding of GIIIA conotoxin to Nav1.4.
We use NavAb crystal structure (ID:3RVY) as a template to create a model of Nav1.4 pore. The sequence of Nav1.4 is noticeably different from NavAb. We have aligned the critical (DEKA) residues forming the selectivity filter with the corresponding (EEEE) residues in 3RVY. Sequences of the four domains of Nav1.4 are aligned with NavAb using ClustalW. A 3D model of the channel is created using Modeller by threading the aligned Nav1.4 sequence for each domain on a corresponding domain of 3RVY. The Nav1.4 model is embedded in a lipid bilayer and simulated for 15 ns. The stable structure of Nav1.4 is used to create the Nav1.4-GIIIA complex. We use HADDOCK for docking and the best complex is selected for refinement by performing 20 ns MD simulations. From the trajectory data, we have determined pair-residue interactions.

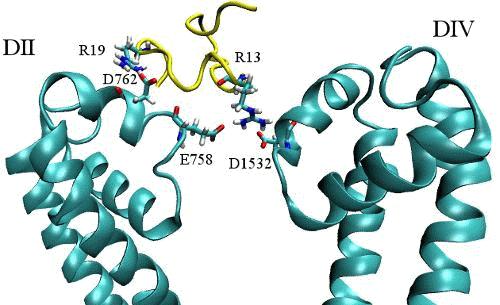
According to the experimental data, R13, K16 and R19 are the most important residues for binding of GIIIA to Nav1.4. Our results show that R13 interacts with E403, E758 and D1532. K16 has stable and strong interaction with D1241 and R19 interacts with D762. This conformation is supported by several mutational data and rationalizes some of the experimental observations; For instance, the critical and unique role of R13 in pore blocking, and the role of domain II in toxin binding.
To provide further validation for the proposed Nav1.4-GIIIA complex, we have calculated binding free energy from the potential of mean force (PMF) of GIIIA. The result reproduces the experimental value within 1 kcal/mol.
In conclusion, the proposed complex of Nav1.4-GIIIA is in good agreement with several mutational data, and its accuracy is further supported by the binding free energy. These comparisons indicate that our model of Nav pore is fairly accurate and provides a useful model for studying toxin interactions with Nav channels.