1. The Raine Study (www.rainestudy.org.au) is a longitudinal Australian birth cohort that has serially assessed the offspring of 2900 pregnant women from 18 weeks gestation in utero to 17 years old.
2. The Raine Study data have shown that low birth weight is a surrogate for poor in utero growth from 18 weeks gestation.
3. A U-shaped relationship between birth size and cardio-metabolic risk exists in this Western Australian cohort, implying that both low and high birth weight are associated with increased risk.
4. High birth weight is a risk factor for cardio-metabolic risk, particularly for females.
5. Lifetime adiposity trajectories are better at predicting metabolic risk of the offspring than birth size or current BMI. Therefore, early life programming is an ongoing process, starting in utero and undergoing at least some level of modification in parallel with changes in adiposity, during early childhood.
6. Maternal smoking during pregnancy, maternal obesity, hypertension and diabetes increase the risk for metabolic risk in the offspring. Breast feeding is protective for cardio-metabolic risk in this Australian cohort.
A large amount of epidemiological data has shown that low birth weight is associated with an increased risk of cardio-metabolic disease since this was first suggested by Barker and colleagues.1,2 It is understood that low birth weight is a surrogate measure for suboptimal intra-uterine environment.3,4 This is substantiated by animal studies showing that restricted in utero environments can induce offspring cardiovascular risk without a change in birth weight.5,6 More recent epidemiological evidence show that high birth weight is also associated with offspring cardio-metabolic risk.7 A few population studies show that both low and high birth weight newborns are at greatest risk (constituting a U-shaped birth weight to cardio-metabolic risk relationship).8,9 Notably this has been shown in what could be classed as transition populations including a modern Asian8 and Pima Indian population.9 We investigated if this relationship held in a modern population that was not in transition.
Early life programming of cardio-metabolic disease is likely to be affected by many genetic and environmental factors in both the mother and offspring. This review focuses on some of these environmental influences with an interrogation of early life programming of cardio-metabolic disease in the Western Australian Pregnancy Cohort (Raine) study. The Raine study comprises a prospective longitudinal cohort following the offspring born to mothers (n=2900) recruited at 14-18 weeks pregnancy between 1989-1992, in Perth, Western Australia. Antenatal ultrasound measurements were taken of the fetus at 18, 24 and 34 weeks gestation. Anthropometry was measured at birth, 1, 2, 3, 5, 8, 10, 14 and 17 years of age. Maternal and offspring lifestyle factors have been recorded at each follow up. Biochemical parameters of the offspring were measured at 8, 14 and 17 years.
The epidemiological literature initially showed that birth weight is linearly and inversely associated cardiovascular risk.1 However, the observations from contemporary populations7 that neonates large for gestational age subsequently develop metabolic syndrome, suggest that additional complexities are likely to exist in this relationship. In fact, two transition populations have shown a U-shaped relationship between birth weight and cardiovascular risk.8,9 To our knowledge, the Raine Study is the first western population to show this U-shaped relationship.10 A recent study in a Japanese population provides further support for a U-shaped birthweight-cardiovascular risk relationship.11 It is likely that historically older cohorts and less affluent populations had lower caloric intake and thus lower prevalence of high birth weight babies.
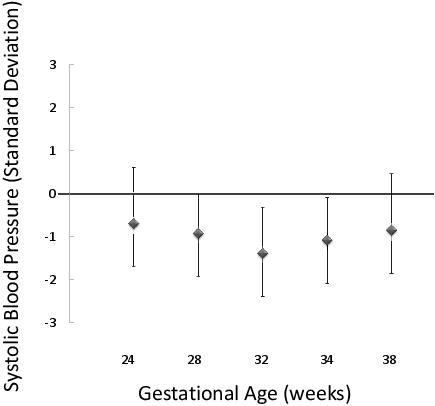
At the 5 year follow-up of the Raine Study, a weak negative relationship between birth weight and offspring blood pressure was identified.12 Further, antenatal femur length independent of the child’s 5 year old height was negatively associated with offspring blood pressure at 5 years of age (Figure 1).13 This relationship between blood pressure and femur length was consistent across gestational ages from 14 to 38 weeks. These findings directly confirm that poor growth in utero is associated with subsequent cardiovascular disease risk.

Figure 1. The relationship between femur length measured by antenatal ultrasound across a range of gestational ages and subsequent systolic blood pressure at 6 years of age in the Raine Study. The parameter estimates and 95% CI for the decrease in systolic blood pressure at age 6 years associated with a one standard deviation increase in femur length, with adjustment for current height is shown. (This figure has been reproduced in modified form from the Journal of Epidemiology and Community Health.13)
The metabolic syndrome is defined in adults using arbitrary cut-points of lipids, waist circumference/body mass index, blood pressure and measures of insulin resistance. Consensus definitions are derived from three major professional bodies, the National Cholesterol Education Program,14 the World Health Organization15 and the International Diabetes Federation.16 As there is no consensus on how to define the metabolic syndrome in childhood,17 the Raine Study, 8, 14 and 17 year reviews utilized two-step cluster analysis18 to define high and low metabolic risk groups.10,19 Cluster analysis does not apply uncertain arbitrary cut-points to define the metabolic syndrome, and has the advantage of being able to identify 2 groups with maximum and minimum intra-individual similarities for the metabolic risk factors.18 Fasting plasma glucose and lipids were measured in 340 Raine participants at the 8 year follow-up, and fasting plasma insulin, glucose and lipids were measured in 1180 and 1053 participants at age 14 and 17 years respectively. These analyses identified 25%,10 29%19 and 18%20 within a “high risk” metabolic cluster at 8, 14 and 17 years, respectively.
At the 8 year follow up, both lowest and highest birth weight quintile neonates were subsequently likely to be identified in the high metabolic risk group, as defined by cluster analysis.10 Compared to the nadir quintile 2, greater metabolic risk was observed in both the lowest and highest quintiles of percentage expected birth weight (Figure 2). Although a U-shaped relationship had previously been observed in populations transitioning to a western lifestyle, this was the first time it had been identified in a fully westernized population.
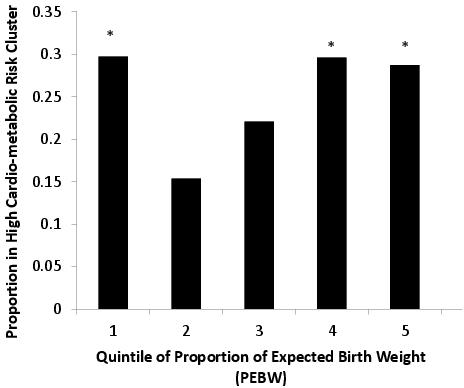
Figure 2. The Proportion of Raine study children at 8 year old follow-up in the “high risk “metabolic cluster (as defined by 2 step cluster analysis) in different percentage expected birth weight quinte (PEBW). *represents P<0.05 compared to the 2nd PEBW quintile (nadir) group. (This figure has been reproduced in modified form from the International Journal of Obesity.10)
At the 17 year old review of Raine participants different relationships between birth weight and cardio-metabolic risk were observed between males and females.20 High birth weight was a strong predictor of risk in females but not males. Sex specific effects have been observed in animal models of fetal programming. In rodents exposure to undernutrition or stressed fetal environments led to hypertension21,22 and neurodevelopmental disorders23 only in male offspring. Potential mechanisms for sex specific effects may lie in the effect of sex hormones which interact with regulatory mechanisms of blood pressure and insulin resistance.21,24 Ojeda et al. performed experiments on a model of intrauterine growth restriction (IUGR) induced by placental insufficiency to explore the developmental origins of adult disease. Estrogen was shown to contribute to normalizing blood pressure in intrauterine growth restricted rodents.24 Testosterone was found to be elevated in adult male IUGR offspring compared to adult male control offspring. Castration completely abolished hypertension in the male IUGR offspring.21 These sex hormones play a role in regulating salt and water homeostasis via the renin angiotensin system.25
One of the major lessons to be learnt from the Raine Study data is that fetal programming of cardio-metabolic risk in a modern western population is caused by two extremes of in utero growth that are manifested as both low and high birth weight. Second, the Raine Study data show that there are sex differences in fetal programming in humans. High birth weight was a strong predictor of cardio-metabolic risk in females but not males. With increasing levels of maternal obesity and gestational diabetes7 in modern society the risk for females is likely to be elevated in the future. Without societal intervention, there is potential for perpetuating this cycle into further generations.
A combination of suboptimal antenatal and postnatal environments is associated with offspring cardio-metabolic disease. It had been argued that postnatal catch-up growth,26 rather than birth weight2 is driving the increased metabolic risk. To investigate this we imputed adiposity trajectories spanning childhood (birth to 14 years).
In a Finish cohort, adults developing cardiovascular disease had growth trajectories characterized by below average early BMI, which then exceeded the average after 11 years of age.27 It was observed that low infant weight gain was associated with increased risk of coronary heart disease. After 12 months of age, rapid weight gain was associated with a further increase in risk.28 Techniques suited for detecting latent growth have confirmed these trajectories and identified alternative obesity trajectories.29-32 In the Raine study, latent growth trajectories were identified using semi-parametric mixed modeling.33 We have identified 7 adiposity trajectories (Figure 3).29 Adiposity z scores are calculated from Center for Disease Control and Prevention growth charts34 expressed as weight for height z score at birth and 1 year and as BMI z score at 2, 3, 5, 8 ,10 and 14 years of age. We showed that adiposity trajectories spanning birth to 14 years are better at predicting insulin resistance and hypertension at age 14 years than birth weight alone or current BMI.29 BMI was strongly associated with cardio-metabolic risk; however, the lifetime pattern (or trajectory) of growth stratifies the risk further. For example, trajectories 1 and 2 have the same BMI at age 14 years, but differ in cardio-metabolic risk. The rising trajectory 2 was associated with greater insulin resistance.29
Rising adiposity trajectories irrespective of whether they originated from low (trajectory 4) or moderate (trajectory 2) birth weight were associated with greater blood pressure and insulin resistance.29 These observations do not abrogate the effect of in utero programming, but do confirm the notion that birth weight is at best an approximate surrogate to represent suboptimal in utero environment. This is consistent with the observations that fetal programming can be induced by altered in utero environment without disturbance in birth size.6,35 An example of fetal programming in humans occurred with the Dutch famine in 1945-46. Offspring of mothers who were exposed to famine before or during pregnancy have greater risk of coronary heart disease,36 obesity,37 and hypertension.38 Epigenetics is likely to be one of the underlying mechanisms mediating this example of fetal programming. Interestingly, epigenetic differences were detected in those of normal birth weight after being exposed to periconceptional starvation.35 Those with exposure to starvation later in gestation did demonstrate reduced birth weight, but in the absence of changes in DNA methylation.35
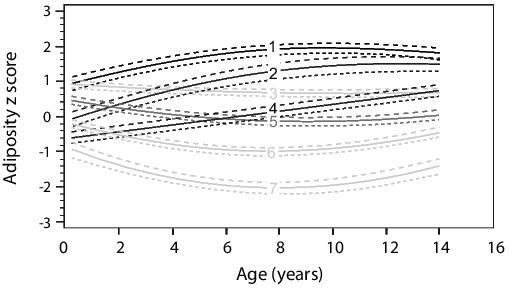
Figure 3. Seven adiposity trajectory groups between birth and 14 years. 1: Stable high (7%). 2: Rising to high (10.2%). 3. Falling to moderate (22%). 4: Rising to moderate (14.4%). 5: Reference group (optimal normal growth) (27.5%). 6: Moderately low stable (15.2%). 7: Very low stable (3.2%). This figure has been reproduced with permission of Diabetes Care.29)
These studies confirm that early life programming is an ongoing process with programming starting in fetal life2 that undergoes at least some level of modification with changes in fatness during childhood.39,40
In Figure 3, latent patterns of growth are illustrated. In particular two rising trajectories, labelled 2 and 4, and a single lifelong high adiposity trajectory labelled 1 were associated with more adverse cardio-metabolic risk. Low birth weight (trajectory 4) and moderate birth weight (trajectory 2) individuals ended up with similar risk factors if the individual has accelerated adiposity during childhood. On the surface, this suggests that birth weight is not an important factor in determining risk and that moderate birth weight should also show increased risk. How is this reconciled to the U-shaped relationships seen in Figure 210 and other studies?8,9 The answer lies in the fact that Figure 2 and similar studies28,41 are measuring the proportion (or relative risk) at each birth weight category for cardio-metabolic risk. While some individuals in trajectory 4 (10%) are of moderate birth weight and develop cardio-metabolic risk, the majority of moderate birth weight fall into trajectories 5 (27.5%) and 6 (15%). Therefore, the relative risk of cardio-metabolic risk at middle ranges of birth weight is still low.
The relative proportions in each of these high risk trajectory groups will influence the birth size to cardio-metabolic risk relationship. As maternal obesity becomes a greater issue, it is likely that the proportions in trajectory 1, skewing cardio-metabolic risk towards high birth weight individuals.
The relationships between birth size and subsequent cardio-metabolic risk provided clues that the in utero environment can “program” the individual for future cardio-metabolic disease. In other words, birth size is acting as a surrogate variable for an intra-uterine suboptimal environment. Therefore, we and others42 have investigated the influence of factors which potentially compromise the in-utero environment upon subsequent cardiovascular risk. These factors have included lifestyle factors (maternal smoking during pregnancy), obstetric complications (such as gestational diabetes7) and risk factors (such as pre-pregnancy body mass index7).
Maternal smoking during pregnancy is related to reductions in birth weight on average 200g.43 In the Avon Longitudinal Study of Parents and Children (ALSPAC) the neonates of mothers who smoked during pregnancy were symmetrically growth retarded and showed catch up growth in first 12 months of life.44 A systematic review showed that all eight studies included showed as increase risk of overweight and obesity related to maternal smoking during pregnancy with odds ratios around 1.5 to 2.0.45 Consistent with this, in the Raine Study maternal smoking during pregnancy was associated with an increased risk of the high risk metabolic cluster (OR=1.82, 95% CI=1.05 to 3.20).10 Babies were then stratified by birth size. Maternal smoking during pregnancy was associated with a further increase in the risk of the metabolic cluster in babies of the highest quintile of birth size (Figure 4). With this stratification by birth weight, the OR related to maternal smoking increased from 1.8 to 14.0 (95% CI=3.8 to 51.1).10
Boney et al. showed that large for gestational age newborns of mothers with gestational diabetes were at significant risk of developing the metabolic syndrome in childhood.7 Even in the absence of a diagnosis of gestational diabetes, obese mothers had a greater chance of having offspring with the metabolic syndrome. In the Raine Study, a “lifelong high adiposity” trajectory consisting of children who were above 1 z score for adiposity from birth to 14 years old were identified. This trajectory was associated with greater blood pressure and insulin resistance and cardio-metabolic risk. It was also associated with increased risk of gestational diabetes, pre-pregnancy maternal obesity and maternal hypertension.29
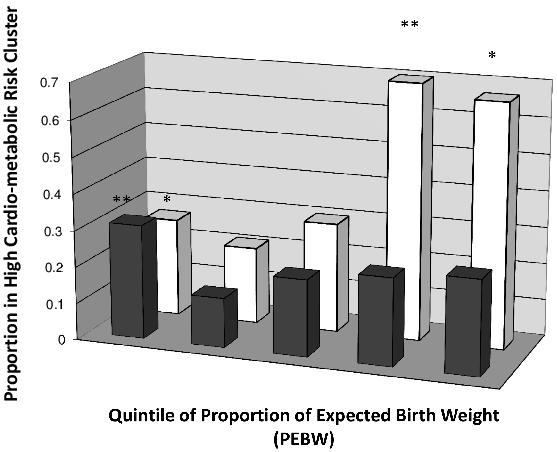
Figure 4. The Proportion of Raine study children at 8 year old follow-up in the “high risk “metabolic cluster (as defined by 2 step cluster analysis) in different percentage expected birth weight quintiles (PEBW), separated by maternal smoking. Solid bars represent children whose mothers did not smoke. Open bars represent the children whose mother’s smoked during pregnancy. **P<0.005, P=0.051 compared to the 2nd PEBW quintile (nadir) group. (This figure has been reproduced in modified form from the International Journal of Obesity.10)
Duration of breast feeding has been shown to be protective (in a dose response fashion) for childhood obesity.46 A systematic review of 70 eligible studies showed that BMI was lower among breast fed subjects.47 Larger studies had smaller effects and in 11 studies factors such as maternal smoking and maternal BMI abolished any detectable effect.47 This implies that publication bias and confounding factors are likely to be at play. Nevertheless, data from the Raine Study showed that breast feeding was also associated with lower global cardio-metabolic risk in the 8 year olds.10 Breast feeding for greater than or equal to 4 months was associated with an OR=0.6 (95% CI=0.37 to 0.97) of the high risk metabolic cluster with clinical features akin to the metabolic syndrome. Individuals belonging to the high risk cluster had greater BMI, systolic blood pressure, waist circumference, fasting triglycerides, homeostasis model of insulin resistance and lower HDL-cholesterol (all P<0.001).10 Breast feeding has also been shown in the Raine Study to be associated with less risk of asthma and other allergic diseases at 5 years of age.48
Randomized clinical trials in adults49 and more recently children50 have shown that omega-3 fatty acids reduce blood pressure and improve lipids profiles. The Raine Study has provided observational data that in male adolescents, omega-3 fatty acid intake is associated with a more favourable cardiovascular risk profile with lower insulin resistance and diastolic blood pressure, and greater HDL-cholesterol.51 In animal studies omega-3 fatty acids have been shown to partly reverse fetal programming of hypertension.52 Further randomized controlled studies will need to be done to ascertain if omega-3 fatty acids can alter early life programming in humans.
Factors that have the potential to ameliorate the risk are of interest as they suggest possible treatment avenues. Thus, certain dietary modifications in childhood may ameliorate childhood metabolic risk as has been shown with breast feeding and childhood omega-3 fatty acid intake in the Raine Study.10,51
Investigations on the Raine Study confirm that fetal programming occurs in a modern, western human population, that it occurs in association with both low and high birth weight and that it is an ongoing process that is substantially modified in childhood. There is a balance of adverse and protective factors at work in early life. Some of these modifiable factors, such as maternal smoking during pregnancy and breast feeding are now the focus of public health programs. Other factors are less readily modified such as maternal hypertension and gestational diabetes. There should be a multi-pronged public health focus to combat adverse fetal programming. Reduction in maternal pre-pregnancy overweight and obesity should be targeted. Concurrently childhood obesity intervention is necessary.
This study was supported by grants from the Australian National Health and Medical Research Council; the University of Western Australia (UWA); the Raine Medical Research Foundation; Healthway Western Australia; the Telethon Institute for Child Health Research; the Faculty of Medicine, Dentistry and Health Sciences of The University of Western Australia; the Women and Infants Research Foundation (UWA); and Curtin University. No potential conflicts of interest relevant to this article were reported. The authors thank all the families that took part in this study and the Raine Study team, which includes data collectors, cohort managers, clerical staff, research scientists and volunteers. Dr Rae-Chi Huang is supported by a UWA Faculty of Medicine Postdoctoral Fellowship.
1. Hales CN, Barker DJ, Clark PM, Cox LJ, Fall C, Osmond C, Winter PD. Fetal and infant growth and impaired glucose tolerance at age 64. BMJ 1991; 303:1019-22.
2. Barker DJP, Hales CN, Fall CHD, Osmond C, Phipps K, Clark PMS. Type 2 (non-insulin-dependent) diabetes-mellitus, hypertension and hyperlipemia (syndrome-X) - relation to reduced fetal growth. Diabetologia 1993; 36:62-7.
3. Barker DJP, Gluckman PD, Godfrey KM, Harding JE, Owens JA, Robinson JS. Fetal nutrition and cardiovascular-disease in adult life. Lancet 1993; 341:938-41.
4. Morley R, Owens J, Blair E, Dwyer T. Is birthweight a good marker for gestational exposures that increase the risk of adult disease? Paediatr. Perinat. Epidemiol. 2002; 16:194-9.
5. McMillen IC, Robinson JS. Developmental origins of the metabolic syndrome: Prediction, plasticity, and programming. Physiol. Rev. 2005; 85:571-633.
6. Rattanatray L, MacLaughlin SM, Kleemann DO, Walker SK, Muhlhausler BS, McMillen IC. Impact of maternal periconceptional overnutrition on fat mass and expression of adipogenic and lipogenic genes in visceral and subcutaneous fat depots in the postnatal lamb. Endocrinology 2010; 151:5195-205.
7. Boney CM, Verma A, Tucker R, Vohr BR. Metabolic syndrome in childhood: Association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics 2005; 115:E290-E6.
8. Wei JN, Sung FC, Li CY, Chang CH, Lin RS, Lin CC, Chiang CC, Chuang LM. Low birth weight and high birth infants are both at an increased risk to have type 2 diabetes among schoolchildren in Taiwan. Diabetes Care 2003; 26:343-8.
9. McCance DR, Pettitt DJ, Hanson RL, Jacobsson LTH, Knowler WC, Bennett PH. Birth-weight and non-insulin-dependent diabetes - thrifty genotype, thrifty phenotype, or surviving small baby genotype. BMJ 1994; 308:942-5.
10. Huang RC, Burke V, Newnham JP, Stanley FJ, Kendall GE, Landau LI, Oddy WH, Blake KV, Palmer LJ, Beilin LJ. Perinatal and childhood origins of cardiovascular disease. Int. J. Obes. 2007; 31:236-44.
11. Sugihara S, Sasaki N, Amemiya S, Kohno H, Tanaka T, Matsuura N. Analysis of weight at birth and at diagnosis of childhood-onset type 2 diabetes mellitus in Japan. Pediatr. Diabetes 2008; 9:285-90.
12. Gurrin LC, Blake KV, Evans SF, Newnham JP. Statistical measures of foetal growth using linear mixed models applied to the foetal origins hypothesis. Stat. Med. 2001; 20:3391-409.
13. Blake KV, Gurrin LC, Beilin LJ, Stanley FJ, Kendall GE, Landau LI, Newnham JP. Prenatal ultrasound biometry related to subsequent blood pressure in childhood. J. Epidemiol. Community Health 2002; 56:713-8.
14. Cleeman JI, Grundy SM, Becker D, Clark LT, Cooper RS, Denke MA, et al. Executive summary of the Third Report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA 2001; 285:2486-97.
15. Alberti K, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications part 1: Diagnosis and classification of diabetes mellitus - Provisional report of a WHO consultation. Diabetic Med. 1998; 15:539-53.
16. Alberti K, Zimmet P, Shaw J. Metabolic syndrome - a new world-wide definition. A consensus statement from the international diabetes federation. Diabetic Med. 2006; 23:469-80.
17. Goodman E, Daniels SR, Meigs JB, Dolan LM. Instability in the diagnosis of metabolic syndrome in adolescents. Circulation 2007; 115:2316-22.
18. Zhang T, Ramakrishnan R, Livny M. BIRCH: A new data clustering algorithm and its applications. Data Min. Knowl. Disc. 1997; 1:141-82.
19. Huang RC, Mori TA, Burke V, Newnham J, Stanley FJ, Landau LI, Kendall GE, Oddy WH, Beilin LJ. Synergy between adiposity, insulin resistance, metabolic risk factors, and inflammation in adolescents. Diabetes Care 2009; 32:695-701.
20. Huang RC, Mori TA, Burrows S, Le Ha C, Oddy WH, Herbison C, Hands BH, Beilin LJ. Sex dimorphism in the relation between early adiposity and cardio-metabolic risk in adolescents. J. Clin. Endocrinol. Metab. 2012; 97:E1014-22.
21. Ojeda NB, Grigore D, Yanes LL, Iliescu R, Robertson EB, Zhang H, Alexander BT. Testosterone contributes to marked elevations in mean arterial pressure in adult male intrauterine growth restricted offspring. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007; 292:R758-R63.
22. Grigore D, Ojeda NB, Alexander BT. Sex differences in the fetal programming of hypertension. Gender Med. 2008; 5:S121-S32.
23. Mueller BR, Bale TL. Sex-specific programming of offspring emotionality after stress early in pregnancy. J. Neurosci. 2008; 28:9055-65.
24. Ojeda NB, Grigore D, Robertson EB, Alexander BT. Estrogen protects against increased blood pressure in postpubertal female growth restricted offspring. Hypertension 2007; 50:679-85.
25. Reckelhoff JF. Gender differences in the regulation of blood pressure. Hypertension 2001; 37:1199-208.
26. Ong KKL, Ahmed ML, Emmett PM, Preece MA, Dunger DB, Avon Longitudinal Study P. Association between postnatal catch-up growth and obesity in childhood: prospective cohort study. BMJ 2000; 320:967-71.
27. Barker DJP, Osmond C, Forsen TJ, Kajantie E, Eriksson JG. Trajectories of growth among children who have coronary events as adults. N. Engl. J. Med. 2005; 353:1802-9.
28. Eriksson JG, Forsén T, Tuomilehto J, Osmond C, Barker DJP. Early growth and coronary heart disease in later life: longitudinal study. BMJ 2001; 322:949-53.
29. Huang RC, de Klerk NH, Smith A, Kendall GE, Landau LI, Mori TA, Newnham JP, Stanley FJ, Oddy WH, Hands B, Beilin LJ. Lifecourse childhood adiposity trajectories associated with adolescent insulin resistance. Diabetes Care 2011; 34:1019-25.
30. Li CY, Goran MI, Kaur H, Nollen N, Ahluwalia JS. Developmental trajectories of overweight during childhood: Role of early life factors. Obesity 2007; 15:760-71.
31. Mustillo S, Worthman C, Erkanli A, Keeler G, Angold A, Costello EJ. Obesity and psychiatric disorder: Developmental trajectories. Pediatrics 2003; 111:851-9.
32. Ventura AK, Loken E, Birch LL. Developmental trajectories of girls' BMI across childhood and adolescence. Obesity 2009; 17:2067-74.
33. Nagin DS. Analyzing developmental trajectories: A semiparametric, group-based approach. Psychol. Meth. 1999; 4:139-57.
34. Ogden CL, Kuczmarski RJ, Flegal KM, Mei Z, Guo S, Wei R, Grummer-Strawn LM, Curtin LR, Roche AF, Johnson CL. Centers for Disease Control and Prevention 2000 growth charts for the United States: improvements to the 1977 National Center for Health Statistics version. Pediatrics 2002; 109:45-60.
35. Heijmans BT, Tobi EW, Stein AD, Putter H, Blauw GJ, Susser ES, Slagboom PE, Lumey LH. Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc. Natl. Acad. Sci. USA. 2008; 105:17046-9.
36. Roseboom TJ, van der Meulen JH, Ravelli AC, van Montfrans GA, Osmond C, Barker DJ, Bleker OP. Blood pressure in adults after prenatal exposure to famine. J. Hypertens. 1999; 17:325-30.
37. Ravelli ACJ, van der Meulen JHP, Osmond C, Barker DJP, Bleker OP. Obesity at the age of 50 y in men and women exposed to famine prenatally. Am. J. Clin. Nutr. 1999; 70:811-6.
38. Stein AD, Kahn HS, Rundle A, Zybert PA, Bruin K, Lumey LH. Anthropometric measures in middle age after exposure to famine during gestation: evidence from the Dutch famine. Am. J. Clin. Nutr. 2007; 85:869-76.
39. Eriksson JG, Forsen T, Tuomilehto J, Winter PD, Osmond C, Barker DJP. Catch-up growth in childhood and death from coronary heart disease: longitudinal study. BMJ 1999; 318:427-31.
40. Parker L, Lamont DW, Unwin N, Pearce MS, Bennett SM, Dickinson HO, White M, Mathers JC, Alberti KG, Craft AW. A lifecourse study of risk for hyperinsulinaemia, dyslipidaemia and obesity (the central metabolic syndrome) at age 49-51 years. Diabetic Med. 2003; 20:406-15.
41. Forsen T, Eriksson JG, Tuomilehto J, Osmond C, Barker DJP. Growth in utero and during childhood among women who develop coronary heart disease: longitudinal study. BMJ 1999; 319:1403-7.
42. Ong KK, Dunger DB. Perinatal growth failure: the road to obesity, insulin resistance and cardiovascular disease in adults. Best Pract. Res. Clin. Endoc. Metab. 2002; 16:191-207.
43. Abel EL. Smoking during pregnancy - a review of effects on growth and development of offspring. Hum. Biol. 1980;52:593-625.
44. Ong KKL, Preece MA, Emmett PM, Ahmed ML, Dunger DB, Team AS. Size at birth and early childhood growth in relation to maternal smoking, parity and infant breast-feeding: Longitudinal birth cohort study and analysis. Pediatric Res. 2002; 52:863-7.
45. Huang JS, Lee TA, Lu MC. Prenatal programming of childhood overweight and obesity. Matern. Child Health J. 2007; 11:461-73.
46. von Kries R, Koletzko B, Sauerwald T, von Mutius E, Barnert D, Grunert V, von Voss H. Breast feeding and obesity: cross sectional study. BMJ 1999; 319:147-50.
47. Owen CG, Martin RM, Whincup PH, Davey-Smith G, Gillman MW, Cook DG. The effect of breastfeeding on mean body mass index throughout life: a quantitative review of published and unpublished observational evidence(1-3). Am. J. Clin. Nutr. 2005; 82:1298-307.
48. Oddy WH, Sherriff JL, de Klerk NH, Kendall GE, Sly PD, Beilin LJ, Blake KB, Landau LI, Stanley FJ. The relation of breastfeeding and body mass index to asthma and atopy in children: A prospective cohort study to age 6 years. Am. J. Public Health. 2004; 94:1531-7.
49. Morris MC, Sacks F, Rosner B. Does fish-oil lower blood-pressure - a metaanalysis of controlled trials. Circulation 1993; 88:523-33.
50. Damsgaard CT, Schack-Nielsen L, Michaelsen KF, Fruekilde MB, Hels O, Lauritzen L. Fish oil affects blood pressure and the plasma lipid profile in healthy Danish infants. J. Nutr. 2006; 136:94-9.
51. O'Sullivan TA, Bremner AP, Beilin LJ, Ambrosini GL, Mori TA, Huang RC, Oddy WH. Polyunsaturated fatty acid intake and blood pressure in adolescents. J. Hum. Hypertens. 2012; 26:178-87.
52. Wyrwoll CS, Mark PJ, Mori TA, Puddey IB, Waddell BJ. Prevention of programmed hyperleptinemia and hypertension by postnatal dietary omega-3 fatty acids. Endocrinology 2006; 147:599-606.