1. Extracellular single neuron recording and labelling studies of primary vestibular afferents in Scarpa’s ganglion have shown that guinea pig otolithic afferents with irregular resting discharge are preferentially activated by 500Hz bone-conducted vibration (BCV) and many also by 500Hz air-conducted sound (ACS) at low threshold and high sensitivity. Very few afferent neurons from any semicircular canal are activated by these stimuli and then only at high intensity.
2. Tracing the origin of the activated neurons shows that these sensitive otolithic afferents originate mainly from a specialized region – the striola – of both the utricular and saccular maculae.
3. This same 500Hz BCV elicits vestibular-dependent eye movements in alert guinea pigs and also in healthy humans. These stimuli evoke myogenic potentials (VEMPs) which are used to test the function of the utricular and saccular maculae in human patients.
4. Although utricular and saccular afferents can both be activated by BCV and ACS, the differential projection of utricular and saccular afferents to different muscle groups allows for differentiation of the function of these two sensory regions. The basic neural data support the conclusion that in human patients in response to brief 500Hz BCV delivered to Fz (the midline of the forehead at the hairline) the cervical VEMP (cVEMP) indicates predominantly saccular function and the ocular VEMP (oVEMP) indicates predominantly utricular function.
5. The neural, anatomical and behavioural evidence underpins clinical tests of otolith function in humans using sound and vibration.
Patients complaining of dizziness need to have assessed the functional state of both semicircular canals and otolith organs in the peripheral vestibular system. The clinical assessment of the semicircular canals involves delivering an angular acceleration to the patient’s head and measuring the eye movement responses. The function of any semicircular canal is now simply tested by using high speed video measures of eye movements during brief, passive unpredictable head rotations. This test is called the video head impulse test.1,2 The otoliths sense linear acceleration, so the standard approach has been to deliver linear accelerations to the patient’s head and measure the responses to that stimulus. Most methods of delivering linear accelerations which have been used - e.g. tilt-chairs, sleds, centrifuges – are excellent devices for research but are not clinically feasible. Recently it has been shown that bone conducted vibration (BCV) of the head causes a series of linear accelerations at the mastoids3 and so BCV is a clinically feasible otolithic stimulus. Supporting this evidence is the demonstration that some otolithic afferents selectively respond to head vibration.4 These results have allowed valid clinical testing of otolith function by measuring the responses evoked by BCV or ACS. The same 500 Hz BCV stimulus which preferentially activates otolith neurons, elicits eye movements in guinea pigs5 and humans.6 Clinical tests measure the EMG preparatory potentials for the eye movement – the so-called vestibular-evoked myogenic potentials (VEMPs). Knowledge of vestibular neural projections to different muscle groups allows these VEMPs to be used as clinical tests of utricular and saccular function.7
There are a host of VEMPs since vestibular input projects to many muscle groups. The two VEMPs which have received the most attention are:
BCV applied to the head causes compression and shear waves to travel through and around the skull and so cause linear accelerations at the mastoids, shown originally by von Bekesy. For example linear accelerometers on the mastoids show that a tap to the midline of the forehead at the hairline (a location called Fz) causes a symmetrical linear acceleration of both mastoids outwards simultaneously.3
To justify the use of these stimuli for clinical evaluation of otolith function, strong evidence is needed about
The evidence has been provided by recent single neuron recording studies4,12,13 and is reviewed briefly below. The methods are described in detail in those papers. The reader is also referred to other recent reviews of this area.12,14-17

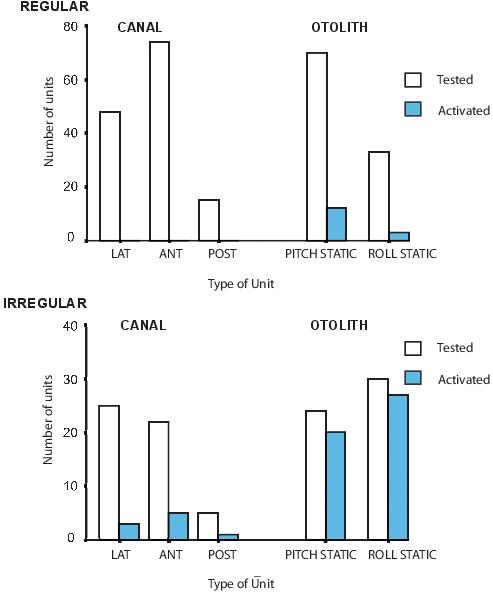
Figure 1. The proportions of neurons tested and activated by BCV stimuli from each vestibular sensory region. Only a very small proportion of semicircular canal neurons, or regular otolithic neurons were activated. However, a large proportion of otolithic irregular neurons were activated. (Reprinted from Experimental Brain Research, Vol 175, Curthoys IS, Kim J, McPhedran SK, Camp AJ, Bone conducted vibration selectively activates irregular primary otolithic vestibular neurons in the guinea pig, pages 256-267, Copyright (2006), with permission from Springer.)
The neural evidence comes from acute experiments12,13 using extracellular single-unit recording from primary vestibular neurons in guinea pigs lightly anaesthetized with intramuscular ketamine (100 mg/kg) and xylazine (4 mg/kg) or rats anaesthetized with 80mg/kg pentobarbital. All procedures were approved by the Animal Ethics Committee of the University of Sydney (protocol numbers L29/4-2010/3/5266 and L29/5-2012/1/5750). The full details of the methods are given in the original papers.12,13
Over 12 years we have recorded from many thousands of vestibular neurons in lightly anaesthetized guinea pigs, and the pattern we reported in 20064 has been confirmed repeatedly (see Figure 1 for a summary of results of neurons activated by BCV). Otolithic afferents with irregular resting discharge are preferentially activated by BCV, whereas irregular semicircular canal neurons rarely respond to 500 Hz stimulation up to the maximum intensity delivered. Regular otolithic or canal neurons are very rarely activated. The conclusion is clear – 500 Hz BCV preferentially activates otolith irregular neurons. Those cells which are activated by 500 Hz vibration tend also to be activated by 500 Hz ACS (Figure 2).13 The average threshold for activation by 500Hz ACS is high – around 120 dB SPL.
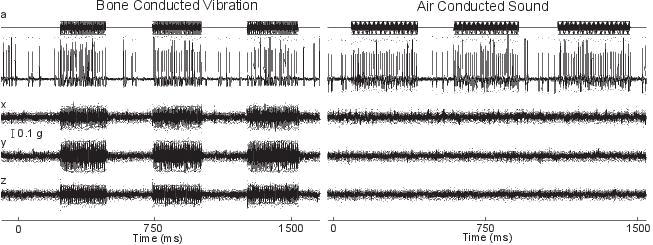
Figure 2. Time series of an irregular otolith neuron during stimulation by BCV and ACS. The top trace (a) shows the command voltage indicating when the stimulus is on. The second trace shows the extracellular recording. The three bottom traces (x, y, z) show the triaxial linear accelerometer recordings of the stimulus. The left panel is an example of BCV stimulation and the right of ACS stimulation of the same neuron, showing that the one neuron is strongly activated by both ACS and BCV. (Reprinted from Experimental Brain Research, Vol 210, Curthoys IS, Vulovic V, Vestibular primary afferent responses to sound and vibration in the guinea pig, pages 347-352, Copyright (2011), with permission from Springer.)
These irregular otolithic neurons have a low threshold and a very steep suprathreshold sensitivity function. The response is not a modest increase to high intensity stimuli requiring many presentations and statistical methods to identify (as used by Zhu et al.18 for ACS in the rat). Instead in these lightly anaesthetized guinea pigs we found large increases in firing to very low intensity vibration stimuli (Figure 3).
We quantified the sensitivity as the percent increase in firing rate relative to the resting discharge as the stimulus strength increased. For those non-responsive neurons, there was no detectable change in response as stimulus strength was increased up to the 6g maximum (Figure 3). In contrast the irregular otolithic neurons which were responsive showed a large increase in firing rate to very small stimuli, so even at 0.1g stimuli there was almost a doubling of firing rate compared to spontaneous levels (Fig. 3). So vestibular neurons form a dichotomy at 500 Hz BCV – most do not respond at all, but the group that does respond, irregular otolithic afferents, shows a very low threshold, highly sensitive response to this stimulation.12,19
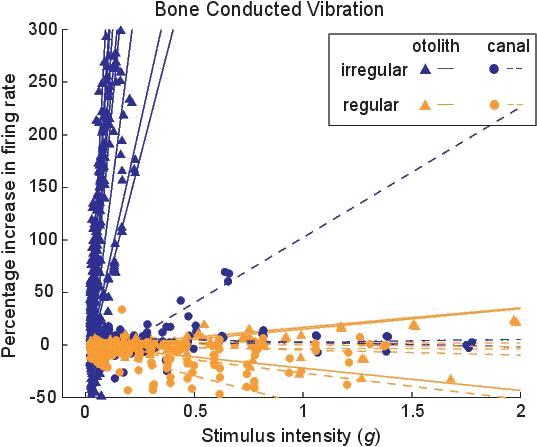
Figure 3. Examples of sensitivity plots of neurons to 500Hz BCV. Each point shows the increase in firing rate as the percentage of baseline firing rate during a single stimulus presentation. The stimulus intensity is calculated in g, root mean square of three axes as recorded by the accelerometers. Each line is the best fit calculation of the responses for one neuron. Reprinted with slight alterations from Experimental Brain Research, Vol 210, Curthoys IS, Vulovic V, Vestibular primary afferent responses to sound and vibration in the guinea pig, pages 347-352, Copyright (2011), with permission from Springer.
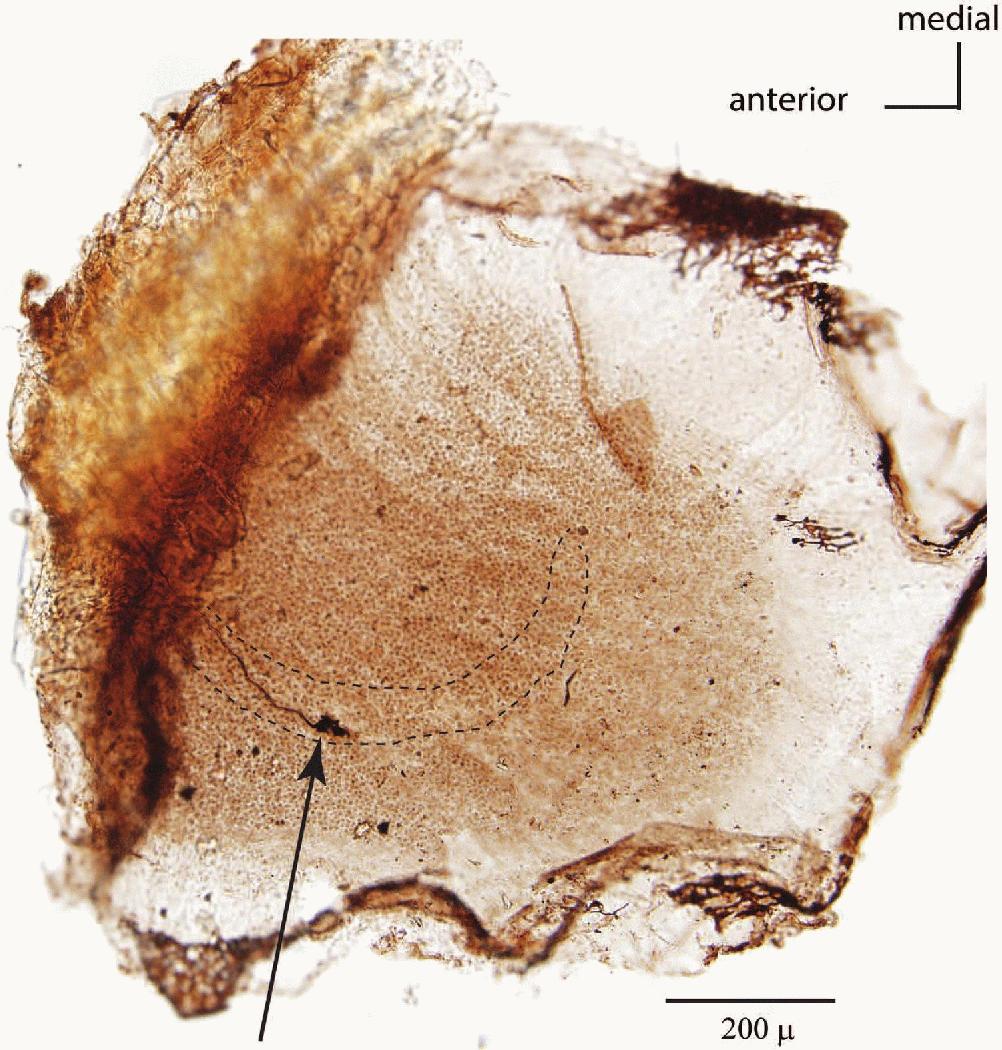
Figure 4. Anatomical evidence that utricular neurons respond to both ACS and BCV. The entire utricular macula has been removed from the temporal bone and processed using immunohistochemistry to show where a primary utricular afferent activated by both ACS and BCV originated from. The point of view is looking straight down on the whole macula. The arrow shows the axon of the activated and neurobiotin labelled neuron, and its termination on receptors in striola of the utricular macula. This result conclusively shows that utricular neurons do respond to both ACS and BCV and so the interpretation in the clinical literature that ACS only activates saccular afferents is not correct. The labelled axon terminates on large calyx endings which envelop the whole type I receptor(s) but in addition there are small bouton endings so this axon is strictly a dimorphic afferent.20 (Reprinted from Brain Research Bulletin, Vol 89, Curthoys IS, Vulovic V, Sokolic L, Pogson J, Burgess AM, Irregular primary otolith afferents from the guinea pig utricular and saccular maculae respond to both bone conducted vibration and to air conducted sound, pages 16-21, Copyright (2012), with permission from Elsevier.)
The neurobiotin labelling showed that the neurons activated by 500 Hz BCV and ACS originated from the striola of either the utricular or saccular maculae and one example is shown in Figure 4. We now have over 50 similar labelled utricular afferents responding to both ACS and BCV and we also have clear anatomical evidence that some afferents activated by ACS and BCV originate from the striolar region of the saccular macula.13 These results show conclusively that both utricular and saccular afferents are activated by both 500 Hz BCV and 500 Hz ACS. In the early clinical literature it had been asserted that ACS only activates saccular afferents in the inferior vestibular nerve; that is simply not correct as Figure 4 shows.
The origin of these otolith irregular afferents from the striola is in agreement with previous evidence. As Goldberg has shown20 otolith irregular neurons terminate mainly on type I receptors in the striola of the utricular and saccular macula, and are especially sensitive to changes in linear acceleration (jerk). That jerk sensitivity may be why these irregular afferents are activated by BCV because 500 Hz BCV consists of 500 rapid changes in linear acceleration (jerks) per second. It is the type I receptors at the striola which are especially vulnerable to the effects of ototoxic antibiotics,21-23 so these are the very otolithic receptors clinicians wish to test. These data complement the evidence that some afferents which were activated by ACS originated from the saccule.24-26
It has been suggested that the response of rat vestibular afferents to ACS implies a major role for horizontal canal afferents in generating VEMPs to ACS. Zhu et al. have reported primary irregular rat semicircular canal neurons which were activated, according to their criterion of activation, by high-intensity ACS.18 The weak activation they found required statistical methods for demonstration, and they judged that a very small increase in the probability of firing to clicks (0.1) constituted activation. Such weak activation is in a different category of response sensitivity compared to the very high sensitivity found for guinea pig otolithic neurons – where neurons showed a response at threshold to stimuli less than 0.1g and had sensitivity factors of an increase in firing of 2000% per g (see Figure 3). However in the Zhu et al. study the physiological evidence also shows that it is otolithic afferents which are preferentially activated by ACS. Zhu et al. state: “The strongest excitations were shown for otolith afferents but some canal afferents were also excited ” (see Zhu et al., 2011,18 page 761). In fact that finding is in agreement with Curthoys et al.4 who found a few irregular semicircular canal afferents activated of the many tested, but that number and their sensitivity paled into insignificance compared to the 83% of irregular otolithic afferents tested which were activated, and activated at low threshold – in some cases below ABR threshold. Zhu et al.18 chose to emphasize the scant number of canal afferents which were activated, but that gives a false picture of the overall response pattern and, as the quote above shows, Zhu et al. also found that it is otolithic irregular afferents which are most strongly excited by ACS. Our unpublished recordings of rat primary vestibular afferents show similar results to the pattern we have reported in guinea pigs.
Afferent input from a very few irregular horizontal canal neurons may contribute to VEMPs but any such contribution will, on the basis of the neural evidence from both the rat and the guinea pig, be very minor. The predominant drive will come not from semicircular canal neurons but from sensitive irregular otolithic afferents.4,12,13
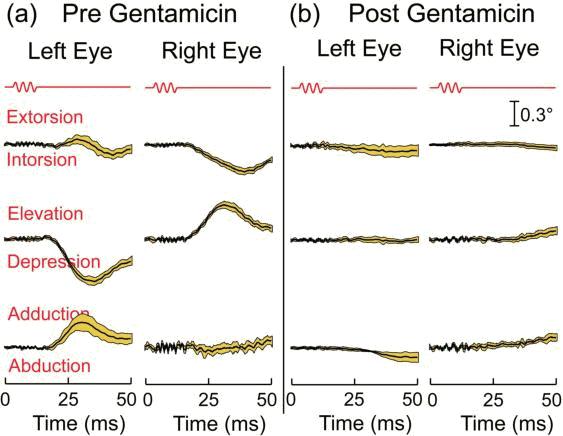
Figure 5. Average eye displacement versus time in response to ten consecutive 500 Hz BCV tone bursts before (a) and after (b) intratympanic gentamicin injection. The first line in red is the command voltage, and it defines stimulus onset and duration. The lines below show the mean and 95% confidence intervals of the responses to the ten successive tone bursts as broken down into three axes. The intratympanic gentamicin almost completely abolishes the eye movement response to BCV, while having very little effect on the auditory brainstem response (ABR). (Reprinted (with some modifications) from a figure in Brain Research Bulletin, Vol 86, Vulovic V, Curthoys IS, Bone conducted vibration activates the vestibulo-ocular reflex in the guinea pig, pages 74-81, Copyright (2011), with permission from Elsevier.)
Suzuki et al.27 showed that high-frequency electrical stimulation of the utricular nerve caused conjugate eye movements in a direction away from the stimulated ear with torsional, vertical and horizontal components – both eyes rolled around the line of sight thanks to the activation of the contralateral inferior oblique and the ipsilateral superior oblique. If 500 Hz BCV acts on the utricular macula then it should cause comparable eye movements in alert guinea pigs (and humans). We tested that hypothesis in guinea pigs by using acute binocular 3d scleral search coils to measure eye movements in response to repeated 7ms 500Hz pulses of BCV to the skull in alert, head-free guinea pigs5 (see Figure 5). The same 500 Hz BCV stimulus which selectively activates otolith neurons elicited eye movements in healthy guinea pigs which, allowing for the lateral placement of the eyes in guinea pigs,28 were similar directions to those reported by Suzuki in cats to electrical stimulation of the isolated utricular nerve. These guinea pig BCV-evoked eye movements were of vestibular rather than proprioceptive or auditory origin for two reasons: 1. they were abolished by bilateral labyrinthectomy (Goodsell 2006, unpublished Honours thesis); and 2. after intratympanic injection of the vestibulotoxic antibiotic gentamicin at levels shown to cause hair cell loss,21-23 there was a reduced eye movement response, although cochlear function remained relatively unaffected, as shown by wave I of the ABR being minimally altered following the intratympanic gentamicin which had substantially reduced the BCV-evoked eye movements (Figure 5).5
In some healthy subjects (without any symptoms of superior canal dehiscence) we used high resolution video eye movement recording to record eye movements in response to 500 Hz BCV, and found that 500 Hz BCV of one mastoid delivered by a small clinical bone oscillator (Radioear B-71) caused small but systematic and reliable eye movement responses with horizontal, vertical and torsional components (Figure 6) at a short latency of about 20 ms or less. In these experiments the subjects were biting on a bite-bar during the BCV stimulation to minimize head rotation and so minimize semicircular canal stimulation.
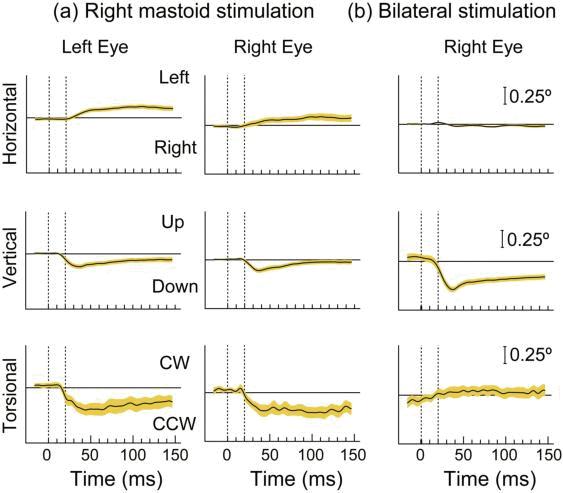
Figure 6. Time series of the 3D eye movement components recorded from the left and right eye in one subject during right mastoid stimulation (a) and bilateral mastoid stimulation (b) with a B71 bone vibrator. Tone bursts of 20ms were presented at 360 ms intervals over a 12 s recording period. Each trace shows the mean values and 95% confidence intervals of 24 individual responses. Horizontal and vertical eye positions (top two panels) were determined by pupil tracking from video images acquired at 180 Hz. The higher noise levels in torsion (bottom panel) resulted from the degradation of the iris striation patterns used to determine torsional eye position due to the trade-off between image quality and the high video acquisition rate. The eye moves horizontally and torsionally away from the side of stimulation during unilateral mastoid stimulation, but there is no clear response in either direction during bilateral stimulation. The eye moves vertically down during both unilateral and bilateral mastoid stimulation, the response being greater during bilateral than unilateral stimulation. (Reprinted (with some modifications) from Annals of the New York Academy of Sciences, Vol 1233, Curthoys IS, Vulovic V, Burgess AM, Cornell ED, Mezey LE, MacDougall HG, Manzari L, McGarvie LA, The basis for using bone-conducted vibration or air-conducted sound to test otolithic function, pages 231-241, Copyright (2011), with permission from John Wiley and Sons.)
The strength of the response and indeed the direction of the eye movement was dependent on the site of stimulation.6 At some stimulus sites on the mastoid, BCV of one mastoid elicits conjugate horizontal movements of both eyes away from the stimulated side, vertical movements of both eyes downwards and torsional movements of both eyes rolling away from the stimulated side (Figure 6), analogous to those found by Suzuki et al., to isolated utricular nerve stimulation in the cat27 and also analogous to those we found with 500 Hz stimulation in the guinea pig.5 At other sites the directions were different as expected because the differently directed linear acceleration will activate different sectors of the utricular macula, and this elicits distinctly different eye movements.29,30 If 500 Hz BCV is applied to both mastoids simultaneously by B-71s, the horizontal eye movement components were cancelled, the torsional components were cancelled but the vertical components summed, so the result is a net downward movement of both eyes.15
This direct eye movement response in guinea pigs and humans justifies the recording of the preparatory EMG potentials as indices of otolithic function. However 500 Hz BCV activates both utricular and saccular afferents but clinicians want separate tests of utricular and saccular maculae. In 2010 Curthoys used the fact that these two sense organs have rather different neural projections to propose a solution.14 There is a very strong projection of utricular afferents to the oculomotor system, whereas the saccular projections to the oculomotor system are weak.31 Conversely saccular projections to cervical spinal regions are strong. So it was proposed that the oVEMP n10 to 500 Hz Fz BCV predominantly tests utricular function and the cVEMP p13-n23 to 500Hz Fz BCV predominantly tests saccular function.14 Although there is excellent evidence for ACS activating saccular receptors and afferents 14 the anatomical and physiological evidence points to the sacculo-ocular pathway being a weak, polysynaptic projection32-34 in contrast to the more robust and direct utriculo-ocular projection.27
To test oVEMPs in the clinic a Bruel and Kjaer 4810 Minishaker can be used to deliver 7ms bursts of 500 Hz at Fz. Fifty presentations of these stimuli at 3/s (even up to 20/s) at moderate intensities (about the strength of a body massager) are not stressful or uncomfortable. The subject must be looking up in order to maximise the oVEMP n10 contribution from the inferior oblique.16
The small excitatory potential, the n10 component of the whole oVEMP, occurs at a latency of around 10 ms, and in response to stimulation at Fz, the n10 beneath both eyes in healthy subjects is about equal.3,10,35 In contrast, in patients after complete unilateral vestibular nerve loss the oVEMP n10 is greatly reduced or absent beneath the eye opposite to the affected ear – the oVEMP is a crossed vestibulo-ocular response.3,10,35 In these unilateral patients there is a large asymmetry of response. These same unilateral patients show a reduced or absent cVEMP p13 on the side of the affected ear – the cVEMP is an uncrossed vestibulo-collic response and on Curthoys’ hypothesis it is an uncrossed sacculo-collic response.
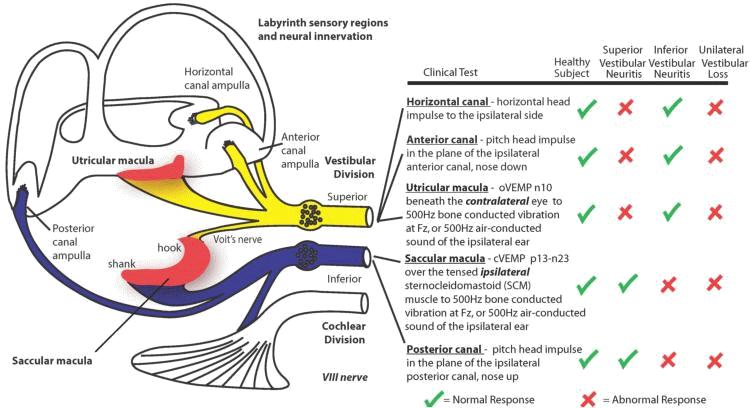
Figure 7. A schematic summary figure relating the clinical test responses to the vestibular receptor structures. The anatomical evidence is from de Burlet.36 oVEMP, ocular vestibular-evoked myogenic potentials; n10, the initial negative potential of the oVEMP response at a latency of around 10 ms; Fz, the location on the forehead in the midline at the hairline; cVEMPs, cervical vestibular-evoked myogenic potentials; p13-n23, the initial positive potential of the cVEMP response at a latency of around 13 ms followed by the initial negative potential at a latency of around 23 ms; SCM, sternocleidomastoid. (Reprinted from Laryngoscope, Vol 122, Curthoys IS, The interpretation of clinical tests of peripheral vestibular function, pages 1342-1352, Copyright (2012), with permission from John Wiley and Sons.)
The projection of otolith neurons is crucial in interpreting clinical otolith tests, and the organization of the peripheral neural projections is especially important since disease (neuritis) may be restricted to individual branches of the vestibular nerve. Afferents from the horizontal and anterior semicircular canals and the utricular macula course in the superior division of the vestibular nerve (Figure 7).36
Most saccular afferents course in the inferior vestibular nerve, except the branch from the small rostral area of the saccular macula (the “hook” of the saccular macula) called Voit’s nerve37 which travels in the superior vestibular nerve. In primates, Voit’s nerve constitutes only about 10% of all the afferents from the saccular macula.38 Figure 8 shows a very simplified schematic of the predominant otolithic central projections and it allows interpretation of the test results.
If the oVEMP n10 is predominantly of utricular origin, then patients with loss of just the superior division of the vestibular nerve should show reduced or absent oVEMP n10 beneath the contralesional eye, but the ipsilesional cVEMP should be unaffected. That result has been demonstrated in a number of separate studies.39-41 Conversely patients with loss of just the inferior division of the vestibular nerve should show reduced or absent cVEMP p13-n23 on the ipsilesional side, but the oVEMP n10 should be unaffected. That prediction has been demonstrated by independent studies.42,43
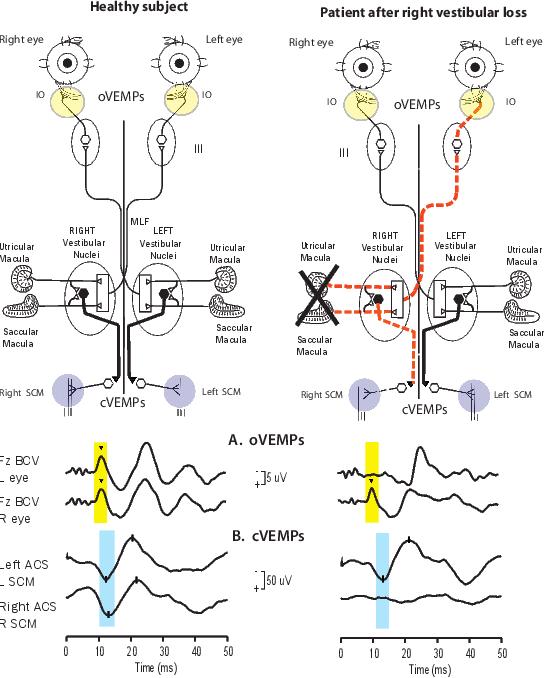
Figure 8. A simplified schematic version of some of the known neural vestibulo-ocular and vestibulo-collic projections that underlie the asymmetric oVEMP and cVEMP responses after unilateral vestibular loss. Electrodes over the inferior oblique (yellow circles) record the oVEMP. Electrodes over contracted SCM muscles (blue circles) record the cVEMP. The projections of the saccular macula in the inferior vestibular nerve, synapsing on an inhibitory neuron in the vestibular nucleus (black hexagon), projecting to spinal motoneurons controlling the SCM, are established by Uchino et al.34 Unilateral vestibular loss results in reduced or absent contralateral oVEMP n10 and a reduced or absent ipsilateral cVEMP p13-n23. (A and B) Examples of averaged EMG responses. oVEMP and cVEMP responses for a healthy subject (column 1), and a patient with right unilateral vestibular loss (column 2). The yellow and blue vertical bands mark the time of healthy oVEMP n10 and cVEMP p13 responses, respectively. The healthy subject displays symmetrical oVEMP n10 responses and symmetrical p13–n23 responses. The uVL patient has normal oVEMP n10 responses beneath the right (ipsilesional) eye and normal p13-n23 responses in the left (contralesional) SCM, and absent oVEMP n10 responses beneath the left (contralesional) eye and absent p13-n23 responses in the right (ipsilesional) SCM. Because oVEMPs are a crossed response and cVEMPs are an uncrossed response, these results are consistent with complete loss of otolithic function on the right side. (Reprinted with slight alterations from Annals of the New York Academy of Sciences, Vol 1233, Curthoys IS, Vulovic V, Burgess AM, Cornell ED, Mezey LE, MacDougall HG, Manzari L, McGarvie LA, The basis for using bone-conducted vibration or air-conducted sound to test otolithic function, pages 231-241, Copyright (2011), with permission from John Wiley and Sons.)
This double dissociation is consistent with the predictions from Curthoys.14 It also points to the conclusion that the contribution of the small bundle of fibres of Voit’s nerve from the rostral saccular macula to the superior vestibular nerve (about 10% of saccular afferents in primates) probably has only a small role (if any) in the generation of oVEMPs.
One prediction from the schema set out above (Figure 7) is that there should be patients with selective utricular loss with all other sensory regions functioning normally, and that has recently been reported.44,45 In this group of patients, specific tests showed the semicircular canals were functional as was the cVEMP, but only the oVEMP was reduced or absent. Luis et al.46 showed dissociation between horizontal canal function and the oVEMP n10 in a patient who lost semicircular canal function temporarily, possibly due to a collapsed horizontal membranous duct. The oVEMP was also lost at this time. After 3 days the horizontal canal function had returned to normal function, but the oVEMP response had not, but did return some weeks later. These data imply that in humans the oVEMP n10 is probably not caused by activation of horizontal semicircular canal afferents, since here there was clear evidence of dynamic canal function, but absent oVEMPs.
There will be many other tests of utricular function developed but at present it is 1) the specific stimulus (500 Hz BCV delivered at Fz) and 2) the specific response (the n10 component of the oVEMP) which are underpinned by the anatomical and physiological evidence, and 3) the results from large patient groups with vestibular losses. It is important to emphasize that the results of clinical tests depend on these specific stimuli and the specific responses, because different stimulus characteristics such as location of the vibrator,47 rise-time and frequencies48 have major effects on the oVEMP n10. Our recent physiological evidence underscores this important point of specificity since we have shown that the selective activation of the otoliths by BCV is lost at low frequencies. In guinea pigs, semicircular canal neurons are activated by 100 Hz BCV, but not by 500 Hz vibration.49
The evidence from guinea pig neurons and eye movements is only indirect support for the interpretations of the human responses to BCV. However this review has shown the very close similarity of the guinea pig and human oculomotor responses to the same stimuli. It is likely that the neural mechanisms in guinea pigs apply to the human results.
We have shown:
Ian Curthoys and Hamish MacDougall are unpaid consultants to Otometrics.
We are grateful for the support of NH&MRC of Australia (grants 632746, 1046826) and the Garnett Passe and Rodney Williams Memorial Foundation.
1. MacDougall HG, Weber KP, McGarvie LA, Halmagyi GM, Curthoys IS. The video head impulse test: diagnostic accuracy in peripheral vestibulopathy. Neurology 2009; 73: 1134-41.
2. MacDougall HG, McGarvie LA, Halmagyi GM, Curthoys IS, Weber KP. The video Head Impulse Test (vHIT) detects vertical semicircular canal dysfunction. PLoS ONE 2013; 8: e61488. doi: 10.1371/journal.pone.0061488
3. Iwasaki S, Smulders YE, Burgess AM, McGarvie LA, Macdougall HG, Halmagyi GM, Curthoys IS. Ocular vestibular evoked myogenic potentials to bone conducted vibration of the midline forehead at Fz in healthy subjects. Clin. Neurophysiol. 2008; 119: 2135-47.
4. Curthoys IS, Kim J, McPhedran SK, Camp AJ. Bone conducted vibration selectively activates irregular primary otolithic vestibular neurons in the guinea pig. Exp. Brain Res. 2006; 175: 256-67.
5. Vulovic V, Curthoys IS. Bone conducted vibration activates the vestibulo-ocular reflex in the guinea pig. Brain Res. Bull. 2011; 86: 74-81.
6. Cornell ED, Burgess AM, MacDougall HG, Curthoys IS. Vertical and horizontal eye movement responses to unilateral and bilateral bone conducted vibration to the mastoid. J. Vestib. Res. 2009; 19: 41-7.
7. Curthoys IS. The interpretation of clinical tests of peripheral vestibular function. Laryngoscope 2012; 122: 1342-52.
8. Colebatch JG, Halmagyi GM, Skuse NF. Myogenic potentials generated by a click-evoked vestibulocollic reflex. J. Neurol. Neurosurg. Psychiatry 1994; 57: 190-7.
9. Halmagyi GM, Yavor RA, Colebatch JG. Tapping the head activates the vestibular system: a new use for the clinical reflex hammer. Neurology 1995; 45: 1927-9.
10. Iwasaki S, McGarvie LA, Halmagyi GM, Burgess AM, Kim J, Colebatch JG, Curthoys IS. Head taps evoke a crossed vestibulo-ocular reflex. Neurology 2007; 68: 1227-9.
11. Rosengren SM, McAngus Todd NP, Colebatch JG. Vestibular-evoked extraocular potentials produced by stimulation with bone-conducted sound. Clin. Neurophysiol. 2005; 116: 1938-48.
12. Curthoys IS, Vulovic V. Vestibular primary afferent responses to sound and vibration in the guinea pig. Exp. Brain Res. 2011; 210: 347-52.
13. Curthoys IS, Vulovic V, Sokolic L, Pogson J, Burgess AM. Irregular primary otolith afferents from the guinea pig utricular and saccular maculae respond to both bone conducted vibration and to air conducted sound. Brain Res. Bull. 2012; 89: 16-21.
14. Curthoys IS. A critical review of the neurophysiological evidence underlying clinical vestibular testing using sound, vibration and galvanic stimuli. Clin. Neurophysiol. 2010; 121: 132-44.
15. Curthoys IS, Vulovic V, Burgess AM, et al. The basis for using bone-conducted vibration or air-conducted sound to test otolithic function. Ann. N. Y. Acad. Sci. 2011; 1233: 231-41.
16. Rosengren SM, Welgampola MS, Colebatch JG. Vestibular evoked myogenic potentials: past, present and future. Clin. Neurophysiol. 2010; 121: 636-51.
17. Rosengren SM, Kingma H. New perspectives on vestibular evoked myogenic potentials. Curr. Opin. Neurol. 2013; 26: 74-80.
18. Zhu H, Tang X, Wei W, Mustain W, Xu Y, Zhou W. Click-evoked responses in vestibular afferents in rats. J. Neurophysiol. 2011; 106: 754-63.
19. Curthoys IS, Vulovic V, Manzari L. Ocular vestibular-evoked myogenic potential (oVEMP) to test utricular function: neural and oculomotor evidence. Acta Otorhinolaryngol. Ital. 2012; 32: 41-5.
20. Goldberg JM. Afferent diversity and the organization of central vestibular pathways. Exp. Brain Res. 2000; 130: 277-97.
21. Hirvonen TP, Minor LB, Hullar TE, Carey JP. Effects of intratympanic gentamicin on vestibular afferents and hair cells in the chinchilla. J. Neurophysiol. 2005; 93: 643-55.
22. Lyford-Pike S, Vogelheim C, Chu E, Della Santina CC, Carey JP. Gentamicin is primarily localized in vestibular type I hair cells after intratympanic administration. J. Assoc. Res. Otolaryngol. 2007; 8: 497-508.
23. Lang H, Liu C. Apoptosis and hair cell degeneration in the vestibular sensory epithelia of the guinea pig following a gentamicin insult. Hear. Res. 1997; 111: 177-84.
24. McCue MP, Guinan JJ, Jr. Acoustically responsive fibers in the vestibular nerve of the cat. J. Neurosci. 1994; 14: 6058-70.
25. Murofushi T, Curthoys IS, Topple AN, Colebatch JG, Halmagyi GM. Responses of guinea pig primary vestibular neurons to clicks. Exp. Brain Res. 1995; 103: 174-8.
26. Murofushi T, Curthoys IS. Physiological and anatomical study of click-sensitive primary vestibular afferents in the guinea pig. Acta Otolaryngol. 1997; 117: 66-72.
27. Suzuki JI, Tokumasu K, Goto K. Eye movements from single utricular nerve stimulation in the cat. Acta Otolaryngol. 1969; 68: 350-62.
28. Simpson JI, Graf W. Eye-muscle geometry and compensatory eye movements in lateral-eyed and frontal-eyed animals. Ann. N. Y. Acad. Sci. 1981; 374: 20-30.
29. Szentágothai J. Die Rolle der einzelnen Labyrinthrezeptoren bei der Orientation von Augen und Kopf im Raume. Akadémiai Kiadó: Budapest. 1952.
30. Szentágothai J. Pathways and synaptic articulation patterns connecting vestibular receptors and oculomotor nuclei. In: Bender MB, (ed.). The Oculomotor System. Hoeber Medical Division. Harper & Row, New York. 1962.
31. Uchino Y, Kushiro K. Differences between otolith- and semicircular canal-activated neural circuitry in the vestibular system. Neurosci. Res. 2011; 71: 315-27.
32. Chan YS, Hwang JC, Cheung YM. Crossed sacculo-ocular pathway via the Deiters' nucleus in cats. Brain Res. Bull. 1977; 2: 1-6.
33. Hwang JC, Poon WF. An electrophysiological study of the sacculo-ocular pathways in cats. Jpn. J. Physiol. 1975; 25: 241-51.
34. Uchino Y, Sasaki M, Sato H, Bai R, Kawamoto E. Otolith and canal integration on single vestibular neurons in cats. Exp. Brain Res. 2005; 164: 271-85.
35. Manzari L, Burgess AM, Curthoys IS. Effect of bone-conducted vibration of the midline forehead (Fz) in unilateral vestibular loss (uVL). Evidence for a new indicator of unilateral otolithic function. Acta Otorhinolaryngol. Ital. 2010; 30: 175.
36. de Burlet HM. Zur Innervation der Macula sacculi bei Säugetieren. Anat. Anz. 1924; 58: 26-32.
37. Voit M. Zur Frage der Verästelung des Nervus acusticus bei den Säugetieren. Anat. Anz. 1907; 31: 635-40.
38. Gacek RR, Rasmussen GL. Fiber analysis of the statoacoustic nerve of guinea pig, cat, and monkey. Anat. Rec. 1961; 139: 455-63.
39. Iwasaki S, Smulders YE, Burgess AM, McGarvie LA, Macdougall HG, Halmagyi GM, Curthoys IS. Ocular vestibular evoked myogenic potentials in response to bone-conducted vibration of the midline forehead at Fz. A new indicator of unilateral otolithic loss. Audiol. Neurootol. 2008; 13: 396-404.
40. Iwasaki S, Chihara Y, Smulders YE, et al. The role of the superior vestibular nerve in generating ocular vestibular-evoked myogenic potentials to bone conducted vibration at Fz. Clin. Neurophysiol. 2009; 120: 588-93.
41. Manzari L, Tedesco A, Burgess AM, Curthoys IS. Ocular vestibular-evoked myogenic potentials to bone-conducted vibration in superior vestibular neuritis show utricular function. Otolaryngol. Head Neck Surg. 2010; 143: 274-80.
42. Manzari L, Burgess AM, Curthoys IS. Ocular and cervical vestibular evoked myogenic potentials in response to bone-conducted vibration in patients with probable inferior vestibular neuritis. J. Laryngol. Otol. 2012; 126: 683-91.
43. Shin BS, Oh SY, Kim JS, Kim TW, Seo MW, Lee H, Park YA. Cervical and ocular vestibular-evoked myogenic potentials in acute vestibular neuritis. Clin. Neurophysiol. 2012; 123: 369-75.
44. Manzari L, Burgess AM, Curthoys IS. Does unilateral utricular dysfunction cause horizontal spontaneous nystagmus? Eur. Arch. Otorhinolaryngol. 2012; 269: 2441-5.
45. Pelosi S, Schuster D, Jacobson GP, Carlson ML, Haynes DS, Bennett ML, Rivas A, Wanna GB. Clinical characteristics associated with isolated unilateral utricular dysfunction. Am. J. Otolaryngol. 2013; 34: 490-5.
46. Luis L, Costa J, Vaz Garcia F, Valls-Solé J, Brandt T, Schneider E. Spontaneous plugging of the horizontal semicircular canal with reversible canal dysfunction and recovery of vestibular evoked myogenic potentials. Otol. Neurotol. 2013; 34: 743-7.
47. Manzari L, Burgess AM, McGarvie LA, Curthoys IS. Ocular and cervical vestibular evoked myogenic potentials to 500 Hz fz bone-conducted vibration in superior semicircular canal dehiscence. Ear Hear. 2012; 33: 508-20.
48. Burgess AM, Mezey LE, Manzari L, Macdougall HG, McGarvie LA, Curthoys IS. Effect of stimulus rise-time on the ocular vestibular-evoked myogenic potential to bone-conducted vibration. Ear Hear. 2013; 34: 799-805.
49. Curthoys IS, Vulovic V, Pogson J, Sokolic L. Responses of guinea pig primary vestibular afferents to low frequency (50-100 Hz) bone conducted vibration (BCV) - the neural basis of vibration induced nystagmus. Society for Neuroscience Abstracts 2012; 574.64. http://www.sfn.org/search?q=574.06+Curthoys