Mapping domains and functional effect of mutations in the skeletal muscle ryanodine receptor calcium release channel
S. Treves1,2
and
F. Zorzato,1,2
1Departments of Anesthesia and Biomedizin, LAB 408 ZLF
Basel University Hospital,
4031 Basel, Switzerland
and
2Department of Life Sciences and Biotechnology
University of Ferrara
44100 Ferrara, Italy.
The skeletal muscle ryanodine receptor (RyR1) calcium release channel is one of the key protein components involved in excitation-contraction coupling, the process whereby an electrical signal (an action potential) is converted into a chemical signal (calcium release) leading to muscle contraction. Both dominant and recessive mutations in RYR1 (the gene encoding the human RyR1) have been identified in different regions of RyR1 (Figure)
and causally linked to a number of neuromuscular disorders, including the pharmacogenetic disorder malignant hyperthermia, and the congenital myopathies Central core disease, Multi-mini core disease, Centronuclear myopathy and Congenital Fiber Type disproportion (Treves et al., 2008).
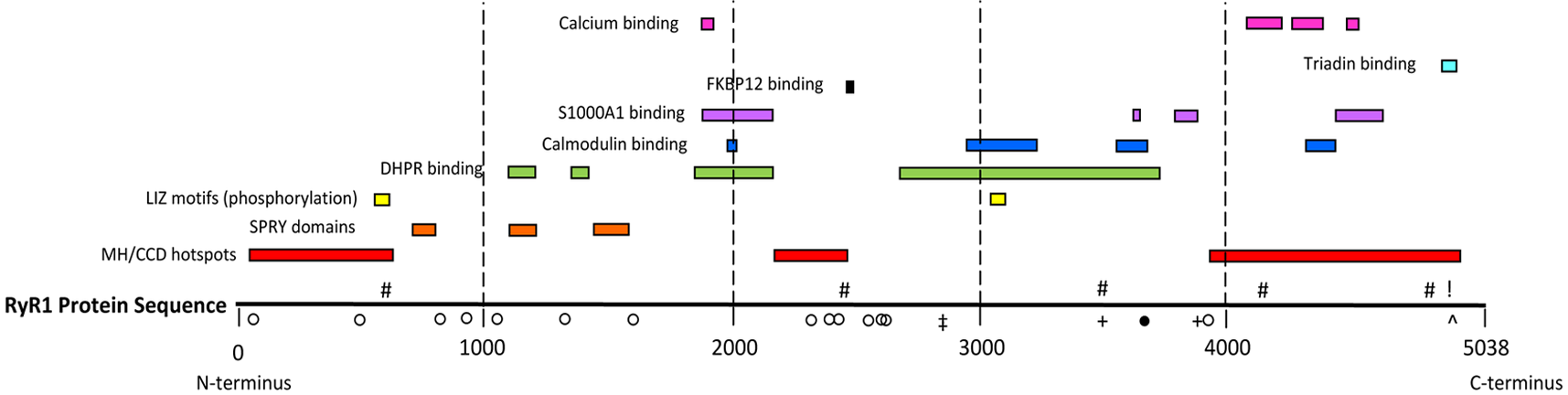
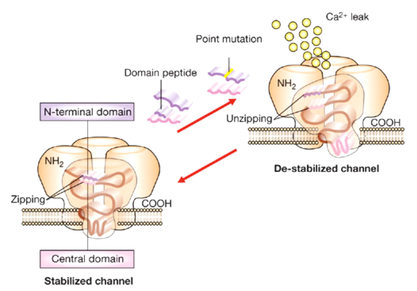
While dominant mutations mostly affect the biophysical properties of the RyR1, the effect of recessive RYR1 mutations is far from clear. Interestingly many mutations identified in patients with ryanodinopathies fall within domains of the RyR1 that have also been found to bind accessory proteins or ionic regulators of the RyR1 calcium channel (Hwang et al., 2012). This aspect linking mutation localization and binding of modulators is important if one is to understand the full impact of a mutation on the calcium channel’s function. Our laboratory has a longstanding interest in clarifying the functional effect of dominant and recessive RYR1 mutations and studying the downstream effects of altered calcium homeostasis in muscle cells obtained from patients affected by neuromuscular disorders. Dominant RYR1 mutations linked to Malignant Hyperthermia and Central Core Disease differently affect intracellular calcium homeostasis as well as calcium dependent signaling pathways. Recessive RYR1 mutations found in some patients with Mulit-minicore disease, Centronuclear myopathy and Congenital Fiber Type Disproportion lead to a significant decrease in RyR1 protein content in skeletal muscle (Treves et al., 2008). Understanding the molecular mechanism leading to RyR1 reduction will be an important step towards the development of therapeutic strategies aimed at improving muscle function in patients with ryanodinopathies leading to protein depletion.
Hwang J.W., Zorzato F., Clarke N. F. and Treves S. (2012) Mapping domains and mutations on the skeletal muscle ryanodine receptor channel. Trends in Molecular Medicine 18, 644-657.
Treves S., Jungbluth H., Muntoni F., Zorzato F. (2008) Congenital muscle disorders with cores: the ryanodine receptor calcium channel paradigm. Current Opinions in Pharmacology 8, 319-326.









