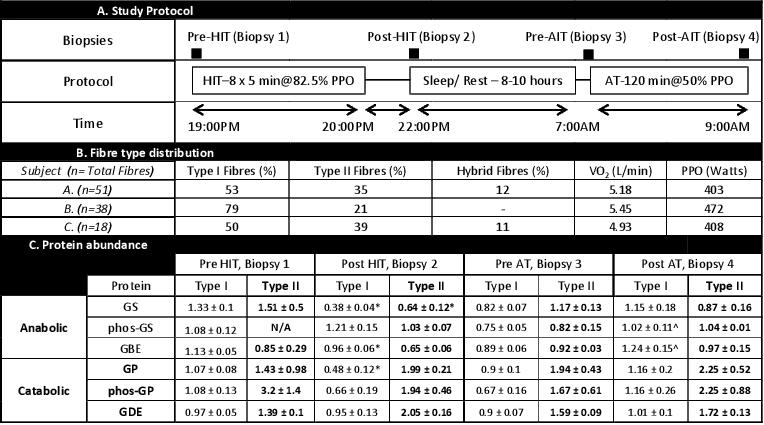
Background: Glycogen is an important fuel source, providing energy for contracting skeletal muscle. Humans possess an active protein/carbohydrate relationship within skeletal muscle. These proteins include anabolic enzymes glycogen synthase (GS) and branching enzyme (GBE), and catabolic enzymes glycogen phosphorylase (GP) and debranhcing enzyme (GDE). Skeletal muscle is heterogeneous, comprising slow-twitch (Type I) and fast-twitch (Type II) fibres, which are distinct in their metabolic and contractile properties. This characteristic is emphasized by the relative abundance of GS, GBE, GP and GDE proteins in rat fast and slow twitch muscle, with a bias for catabolic proteins in fast-twitch fibres. In the current study we used two exercise interventions (Table A) with differential demands on glycogen utilization, and single fibre western blotting to assess the abundance of glycogen-related proteins in specific fibres in endurance trained cyclists.
Methods: The study was approved by the Human Ethics Committee at RMIT University. Muscle biopsies were taken from the vastus lateralis of 3 trained cyclists using the Bergstrom biopsy technique. Following injection of 1% lidocaine (Xylocaine) into the skin/fascia, a small incision was made, and muscle samples were collected at four time points (Table A). Individual fibres were collected from freshly obtained tissue under paraffin oil, and prepared for western blotting analysis.
Results: Fibre type distribution varied between subjects (Table B). Following HIT, there was a decrease in GS, GBE, GP, but not GDE in Type I fibres, but only in GS in Type II fibres (Table C). Following AT only phos-GS and GBE and this was in Type I fibres only. This shows altered adaptions to the opposing exercise bouts.
Conclusion: Glycogen regulation is complex, and fibre type needs to be considered when understanding regulation. Any analyses conducted in whole muscle should therefore be interpreted with caution.
