Aging is associated with a decline of most physiological systems of the body, resulting in a decline in physical and exercise capacity. Skeletal muscle experiences critical alterations with aging, such as muscle atrophy (sarcopenia), loss of oxidative enzyme activity and decreased muscle capillarity, which eventually lead to decreased muscle endurance. Muscle capillaries, particularly, play critical roles in muscle metabolism and performance. Aging is a dominant risk factor for muscle capillary density maintenance because of age-associated impairment in angiogenesis and escalation in endothelial dysfunction. Reduction in skeletal muscle microvasculature has also been reported to be a contributing factor for exercise intolerance in class I-III chronic heart failure and peripheral arterial disease. It is well established that regularly performed endurance exercise increases capillary density in skeletal muscle. However, muscle neovascularization in response to acute exercise is highly attenuated in aged compared to young men and women. This implies that aged individuals are severely compromised to achieve full health benefits of exercise-induced angiogenic adaptations of skeletal muscle tissue. Therefore, identification of a therapeutic target that can ameliorate age-associated decline in vascular density and exercise capacity will be clinically valuable.

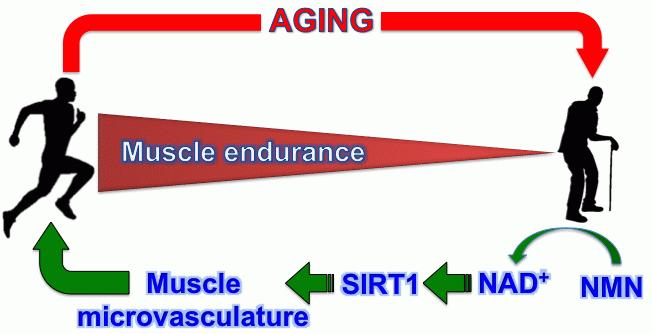
Sirt1, an NAD+-dependent protein deacylase, has recently emerged as an important regulator of angiogenesis and endothelial dysfunction. Genetic ablation of Sirt1 abrogates postnatal vascular growth in vivo. In hindlimb ischemia model, Sirt1 deletion results in the impairment of skeletal muscle capillary growth. Most importantly, Sirt1 activation through pharmacological interventions has been a useful approach to reproduce the benefits imparted by it, including protection from diabetes, metabolic disorders, cardiovascular disease and neurodegeneration. While several Sirt1 activating compounds (STACs) have been identified that stimulate Sirt1 activity via allosteric mechanism, bioavailability of NAD+ cofactor remains a critical determinant of Sirt1 activity in aged and several diseases. During aging, NAD+ levels significantly decrease in several tissues in mammals. Boosting the levels of NAD+ using NAD+ precursor, such as nicotinamide mononucleotide (NMN), is emerging to be a promising approach to ameliorate metabolic abnormalities and age-related pathologies. The present study investigates if Sirt1 plays any role in remodeling muscle microvascular density and if NMN can be used to rescue age-associated decline in muscle microvascular density and exercise capacity.
We, hereby, report that we have identified Sirt1 as a critical regulator of skeletal muscle microvascular remodeling during aging and exercise. To this end we have generated endothelial cell-specific Sirt1 knockout and overexpressing mice to study the role of Sirt1 in muscle capillaries in vivo. Our data showed that Sirt1 loss-of-function in murine muscle capillaries resulted in 50% decrease in muscle capillary density by 6 months of age. This eventually led to a decline in exercise capacity in these mice. Using exercise-mimetic mice that have muscle-specific PGC1α overexpression, we have demonstrated that Sirt1 in endothelium is required for PGC1α-mediated angiogenesis in muscle. Moreover, we showed that endothelial-specific Sirt1 deletion in these mice reduced their exercise capacity in spite of PGC1α overexpression. Subsequently, we showed that gain-of-function of Sirt1 in endothelial cells prevent age-associated decline in muscle capillary density and exercise capacity in mice. After confirming the role of endothelial Sirt1 in muscle microvasculature remodeling and exercise capacity we investigated the possibility of reproducing these beneficial effects Sirt1 by pharmacologically raising NAD+ levels in old mice. We supplemented NMN at 300mg/kg/day in the drinking water of 18 months old chow-fed mice. After two months of NMN feeding, we observed a robust improvement in exercise capacity of old mice, where NMN-fed old mice ran two times greater distance compared to vehicle-fed old mice in a treadmill test. We also observed that systemic administration of NMN reversed the age-associated decline in muscle capillary density, where NMN-fed mice have higher capillary density compared to vehicle-fed mice. Next we validated the role of NMN and its Sirt1-dependency in regulating endothelial function using ex vivo and in vitro angiogenesis assays. Results from these assays confirmed that Sirt1 regulates NMN-mediated pro-angiogenic properties in endothelial cells. Identifying the therapeutic role of NMN in modulating vascular density will be of high clinical significance in the treatment of diseases with vascular insufficiency such as peripheral artery disease, myocardial ischemia, vascular disease of the central nervous system, erectile dysfunction, and wound healing.