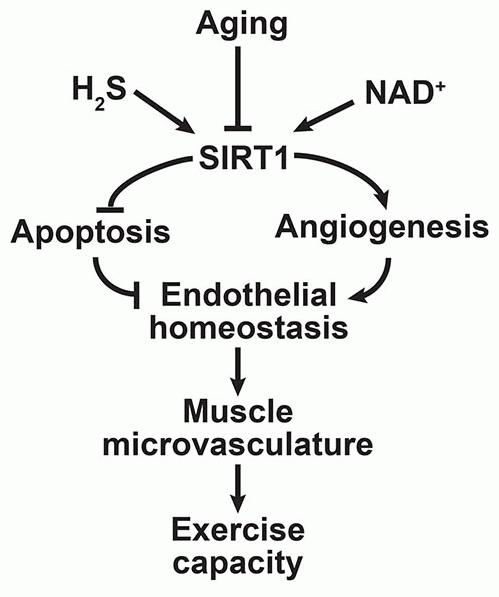
In skeletal muscle, endothelial cell dysfunction, impaired microcapillary formation and a progressive decline in exercise capacity are hallmarks of aging, yet the underlying causes are poorly understood. SIRT1, an NAD+-dependent protein deacylase, is a pro-longevity factor that mediates many of the health benefits of caloric restriction. Metabolic precursors of NAD+ have attracted attention for their ability to reverse the decline in NAD+ during aging, improve health, and extend lifespan in mice. Here, we show that deletion of the SIRT1 gene specifically in endothelial cells results in an accelerated loss of capillary density and exercise capacity during aging along with an inability to respond to exercise. Conversely, overexpression of SIRT1 in endothelial cells increases capillary density and maintains the endurance capacity of old mice. Treatment of old mice with the NAD+ precursor nicotinamide mononucleotide (NMN) restores NAD+ levels to those of young mice, with a concomitant SIRT1-dependent increase in muscle capillary formation and exercise capacity. Hydrogen sulfide gas (H2S) extends lifespan in simple metazoans and is implicated in mediating the benefits of dietary restriction in mammals possibly by activating SIRT1. We show that co-treatment of NMN and the H2S donor sodium hydrosulfide (NaHS) further improved the muscle capillary density and exercise capacity in aged mice by activation of endothelial SIRT1. These data indicate that a decline in endothelial SIRT1 activity underlies capillary loss in muscle and exercise capacity with aging, effects that appear to be reversible by raising levels of NAD+ and H2S in the endothelium.
