Cyclic neural motor patterns in the full isolated intact intestine of the mouse
L.J. Keightley,1
L. Wiklendt,1
J.W. Arkwright,2
T.J. Hibberd,1
P.G. Dinning,3
S.J.H. Brookes,1
N.J. Spencer1
and
M. Costa,1
1Department of Physiology,
School of Medicine,
Flinders University,
SA 5042, Australia,
2Computer Science,
Engineering and Mathematics,
Flinders University,
SA 5042, Australia
and
3Department of Gastroenterology and Surgery,
Flinders Medical Centre,
Flinders University,
SA 5042, Australia.
Gastrointestinal motility is largely controlled by the enteric nervous system acting on pacemaker driven smooth muscle activity. Neurally-dependent cyclic motor activity has been recorded in the mouse colon and was originally called "colonic migrating motor complexes" (CMMC; Spencer, 2001). In the mouse small intestine, similar cyclic activity has also been recorded in vitro, and was called "intestinal migrating motor complexes" (IMMC; Bush et al. 2000). In this work we have recorded intraluminal force from the full intact small and large intestine of mice, kept constantly distended by custom made miniature fiberoptic manometric catheter of 3.0 mm diameter, developed and built at CSIRO (CSIRO, Materials Science and Engineering, Lindfield, NSW) Flinders by one of the authors (JA). 78 adult male and female mice (C57BL/6J) were used. The animals were killed humanely according to Flinders Animal Welfare Committee guidelines. The entire intestine was removed and placed in an organ bath with carbogenated Krebs solution at 36°C. The natural contents were allowed to empty spontaneously before the miniature manometric catheter was introduced gently from the oral end. Pressure sensors, located at 10 mm intervals, gave multiple traces of isometric force, which were plotted either as traces or as pressure maps (Pmaps) (see Figure a).
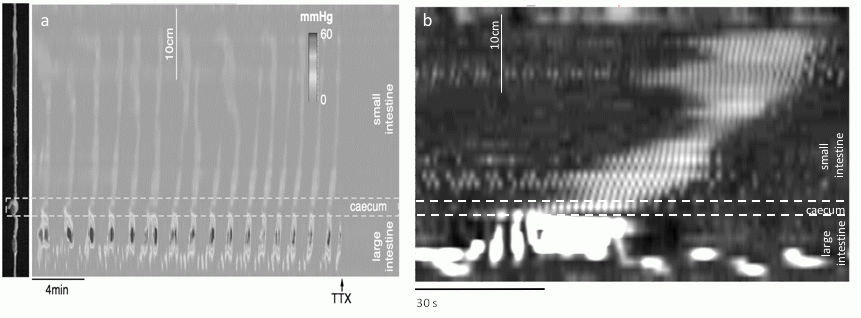
Following equilibration, cyclic motor complexes (CMC) were recorded. In the small intestine they occurred at 0.50±0.13 SD cpm (n=26) and consisted of clusters of phasic peaks at 43.3±3.3 SD cpm extending over 14.01± 3.32 cm. They propagated in both directions, 58% aborally, 40% orally and 2% appearing simultaneously along a length of intestine. In the colon similar cyclic activity occurred at 0.57±0.14 SD cpm consisting of cluster contractions at 19.7±2.0 SD cpm. The colonic motor complexes preferentially (70%) propagated in the aboral direction, 23% propagating orally and the remaining 7% with no apparent direction. Strikingly, in 4 out of 37 experiments cyclic motor complexes propagated continuously across the cecum in either direction occupying the entire small and large intestine (see Figure b). Other preparations showed clusters of smaller peaks that constitute the cyclic motor complexes in the small intestine. All CMC in both small and large intestine were blocked by TTX (0.6μM), hyoscine (1μM) or by hexamethonium (100μM). Physostigmine (0.3 μM) did not significantly affect the CMCs. These results show that continuous distention of the isolated intestine elicits neurally-dependent cyclic motor complexes which involves excitatory enteric cholinergic motor neurons and propagation along the entire intestine mediated by nicotinic interganglionic transmission. After neural blockade, no evidence for low frequency spontaneous myogenic motor activity was seen. The role of CMCs in propulsion remains to be established.
Bush TG, Spencer NJ, Watters N, Sanders KM, Smith TK. (2000) Auton Neurosci Basic Clin 84, 162–168.
Spencer NJ. (2001) Curr Opin Pharmacol 1, 604–610.








