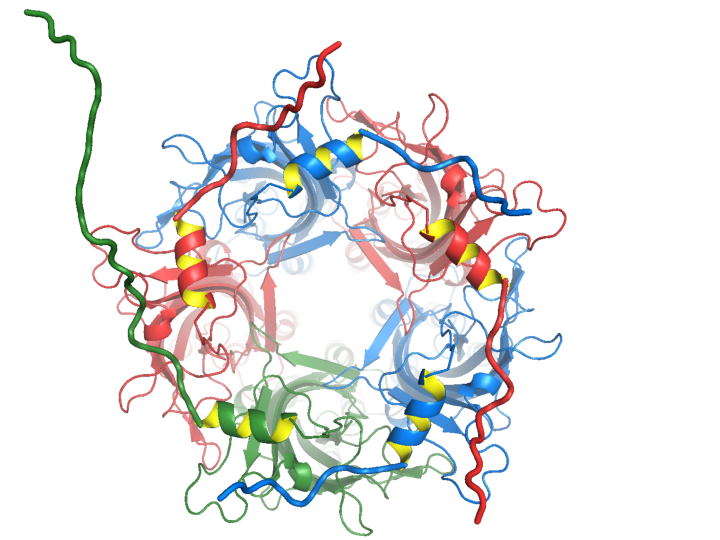
Members of the pentameric ligand-gated ion channel superfamily (pLGICs) mediate both excitatory (eg. nicotinic acetylcholine receptors (nAChRs), and inhibitory (eg. GABAA/C receptors (GABAA/CRs)) fast synaptic transmission in the central nervous system. Recently resolved pLGIC structures show an α-helix near the N-terminus of eukaryotic receptors. Helices are not present at this position in prokaryotic homologs, however, implying that these helices may not be structurally or functionally essential. In GABAA/CRs, these helices are preceded by 8-36 additional residues, which we term the N-terminal extension, not present in nAChRs. These extensions are located at subunit interfaces where they may be important for inter-subunit interactions.
As previously shown for α7 homomeric nAChRs, we found that the N-terminal α-helix is functionally essential in homomeric GABACRs and that the N-terminal extension contributes to receptor assembly and trafficking. The figure shows α1β2γ2 GABAAR model from the extracellular side, with subunits coloured red-α1, blue-β2 and green-γ2, and yellow internal highlighting of N-terminal α -helices. N-terminal extensions, shown as thicker tubes, are modelled as random coils to indicate their length. Conversely, in heteromeric α1β2γ2 GABAARs we found that the role of the N-terminal α-helix was highly subunit dependent, being functionally dispensable in β2 or γ2 subunits but being important in the α1 subunit for assembly and trafficking. This striking subunit dependence continued in the N-terminal extension, with deletions here in the α1 subunit markedly assembly and trafficking but again having little effect in the β2 or γ2 subunits. Finally we found that small differences in the N-terminal extensions of the β2 or β3 subunits differentially modulate the functional effects of an epilepsy-linked mutation in the γ2 subunit. Thus, our data support a role for the N-terminal regions in pLGIC assembly trafficking and function, with these roles being more specialized to different subunits in heteromeric receptors.
