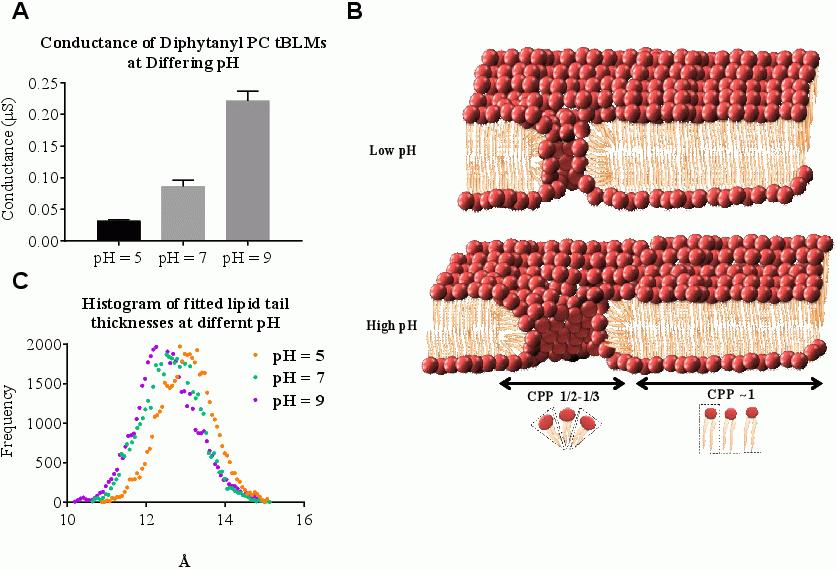
We report that increasing the H3O+ concentration when lowering the pH reduces the intrinsic ionic conduction through phospholipid bilayers (Fig 1A), which is counter to what might be expected from increasing the H3O+ concentration. We attribute the conduction decrease to a decrease of the molecular area per lipid. These effects are seen at H3O+ concentrations in the range nM to μM despite these being very low concentrations compared to that of a typical bathing electrolyte solution of 135mM. The reason for the potency of the H3O+ ion is the high electric field gradients generated by the small size of the ion. These high field gradients make it a significant factor in determining the integrity of lipid bilayers. To describe these effects we use the critical packing parameter (CPP) = v/(a0l) (Israelachvili, Marcelja & Horn, 1980). Here v is the molecular volume, a0 is the molecular area at the oil/water interface and l is the length of the hydrocarbon chain wthin the assembled organisation. For planar geometries CPP = 1. For micellar geometries CPP=1/3. We present a model in which the pH dependent reduction in molecular area reduces a0 favouring a slight increase in CPP causing membrane lipids to migrate from highly curved defects (toroidal pores) already present in the membrane (Fig 1B), reducing the pore diameter. To support this model, we provide evidence of the effects of the hydronium ion on lipid geometry using neutron reflectometry (Fig 1C). In addition these geometrical constraints are likley to influence the lipid composition of membranes in organisms that have evolved in environments of widely differing pH. Those that evolve in highly acidic volcanic vents are predicted to possess shorter chain lipids than those that evolved in highly alkaline salt lakes, explaining these observed differences in nature (de Rosa et al., 1983: Mykytczuk et al., 2010). The impact of the H3O+ ion concentration on the hydrogen bonding within the polar groups of lipid membranes provides an explanation for the actions of some non-pore forming antimicrobial peptides on lipid bilayers.
de Rosa M, Gambacorta A, Nicolaus, Grant W.D. (1983) Microbiology 129: 2333-2337.
Israelachvili JN, Marcelja S, Horn RG. (1980) Q Rev Biophys 13: 121-200.
Mykytczuk NCS, Trevors JT, Ferroni GD, Leduc LG. (2010) Extremophiles 14: 427-441.
