Introduction. The lymphatic system comprises a network of vessels, nodes and lymphoid tissue distributed throughout the body which has important functions in fluid balance, lipid absorption and immune regulation (Trevaskis et al., 2015). Historically the brain has been considered an organ devoid of lymphatic vessels. However, recently, Aspelund et al., (2015) and Louveau et al., (2015) provided evidence that tracers and immune cells drain from the brain via lymphatic vessels in the meninges that connect to the cervical lymph vessels and nodes in the neck. This opened a new research field to study the role of the lymphatics in health and disease of the brain which will require new methods to evaluate transport from the brain via the lymphatics.
Aim. To develop a new method to cannulate and collect cervical lymph in rats and to analyse the composition of cervical lymph in rats for the first time.
Methods. A novel method was developed to cannulate the cervical lymph duct in rats. Briefly, adult male Sprague-Dawley rats (n=4) were anaesthetized using 5% isoflurane gas delivered via nose cone and the cervical lymph trunk and carotid artery on one side were cannulated. Lymph and blood samples were collected for up to 8 hours. Lymph flow rate was measured and lymph and blood composition of lipids, proteins and immune cells were analysed using commercial kits, ELISA or flow cytometry.

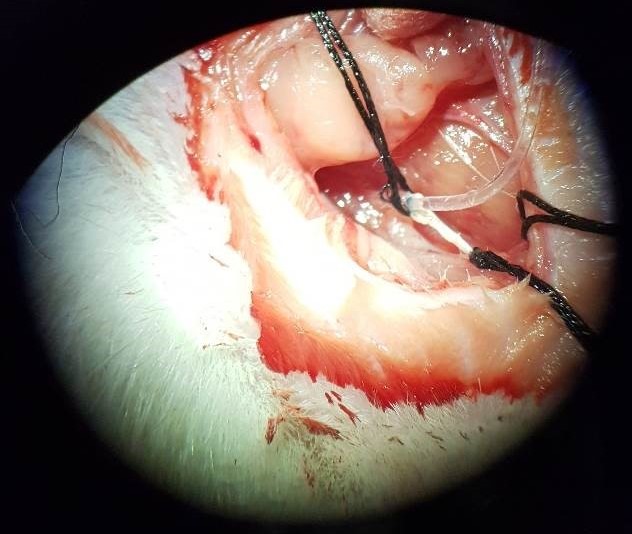
Results and Discussion. A method was successfully developed to cannulate and collect rat cervical lymph as shown in the Figure. The cannulation was difficult due to anatomical variability in lymph duct size and branching and issues related to maintaining cannula patency. Assays of the collected lymph found mean total protein and triglyceride concentrations of 9.6 ± 3.3 mg/mL and 0.5 ± 0.2 mg/mL (mean ± SD), respectively. The triglyceride concentration was lower than those reported in mesenteric lymph (2.7 mg/mL by Ockner et al., 1969), which is likely due to the additional contribution of dietary lipids that are transported from the intestine via the mesenteric lymph. The mean lymph:plasma (L/P) protein ratio was found to be 0.4 ± 0.1 for collected cervical lymph. The L/P protein ratio for cervical lymph is expected to be lower than those reported for liver lymph (0.68 by Barrowman & Tso, 2010) due to the sinusoidal, fenestrated endothelium of hepatic blood vessels facilitating increased transport of proteins from the plasma into lymph. The mean flow rate was 0.05 mL/h/kg, which is similar to the flow rate previously described in rabbits (0.04-0.08 mL/h/kg) (Bradbury & Westrop, 1983) but less than in larger animals such as sheep (0.3 mL/h/kg) (Boulton et al., 1998). Most (>99%) of the cells in the cervical lymph were CD4+ and CD8+ lymphocytes.
Conclusion. As far as we are aware this is the first description of a method to collect and analyse the composition of cervical lymph from rats. The method will enable future studies to evaluate the role of the lymphatics in the transport of different molecules (solutes, drugs etc.) and cells from the brain and to determine the impact of factors such as age and disease on lymphatic clearance from the brain.
Aspelund A, Antila S, Proulx S, Karlsen TV, Karaman S, Detmar M, Wiig H, Alitalo K. (2015) J Exp Med, 212(7): 991-9
Barrowman JA, Tso P. (2010) In: Comprehensive Physiology, ed Pollock DM. pp. 1733-1777. John Wiley & Sons, Inc.
Boulton M, Flessner M, Armstrong D, Hay J, Johnston M. (1998) Am J Physiol, 274(1 Pt 2): R88-96
Bradbury MW, Westrop RJ. (1983) J Physiol, 339: 519-34
Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske J, Derecki NC, Castle D, Mandell JW, Lee KS, Harris TH, Kipnis J. (2015) Nature, 523(7560): 337-41
Ockner RK, Hughes FB, Isselbacher KJ. (1969) J Clin Invest, 48(11): 2079-88
Trevaskis NL, Kaminskas LM, Porter CJ. (2015) Nat Rev Drug Discov, 14(11): 781-803