
Contents




|
Programme
Contents 


|
Antimicrobial peptides (AMPs) are a key component of the innate immune defense of all forms of life, but the mechanisms driving their antimicrobial activity are only poorly understood. AMPs are known to preferentially bind to microbial membranes and form pores. However, how AMPs selectively target microbial membranes and the relationship between pore-formation and antimicrobial activity remains largely undetermined. This is chiefly due to the lack of atomic detail structural information detailing the mechanisms of membrane binding and channel formation, as well as the pore architecture.
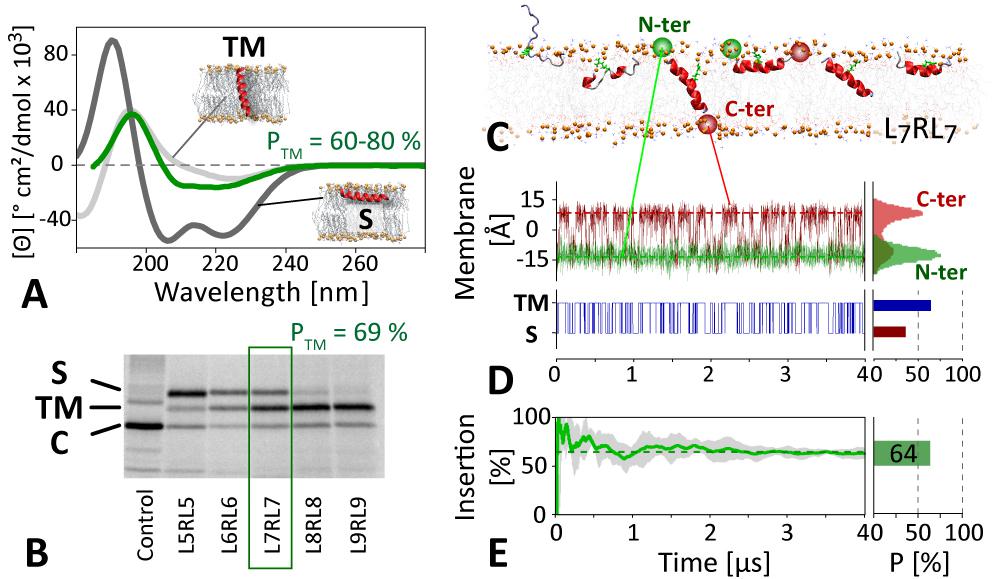
Previous attempts at determining the structures of AMP channels have been thwarted by the transient nature of AMP channels. Here we demonstrate and experimentally validate a new methodology, based on unbiased atomic detail equilibrium molecular dynamics simulations, that allows direct ab initio assembly of functional AMP pores. The figure shows oriented CD (A) and in vitro partitioning experiments (B) provide thermodynamic averages of the insertion propensity of a peptide (GL7RL<7G). Unbiased MD simulations reveal that the native state is an ensemble of surface bound (S) and transmembrane configurations (TM), and provide additional information on the atomic detail structural ensemble, membrane insertion mechanism (C), kinetics (D), and thermodynamics (E), which are in quantitative agreement with the experiments (PTM = 70±10%). This approach accurately predicts the insertion mechanisms and native state structures of membrane active peptides at atomic resolution, accurately reproducing experimental ensemble averages and partitioning data synchrotron radiation circular dichroism spectroscopy and in vitro translocon experiments (Ulmschneider et al., 2011; Ulmschneider et al., 2014). The insertion probability as a function of peptide length follows two-state Boltzmann statistics and reveals atomic-resolution details about the partitioning process. Here we demonstrate the further extension of this methodology to capture the spontaneous assembly of peptides into functional channels in the bilayer.
Ulmschneider JP, Smith JC, White SH, Ulmschneider MB. (2011) J Am Chem Soc 133: 15487–15495.
Ulmschneider MB, Ulmschneider JP, Schiller N, Wallace BA, von Heijne G, White S. (2014) Nature Commun 5: 4863.