
Contents




|
Programme
Contents 


|
Background: The importance of glycogen as a fuel source in skeletal muscle is irrefutable, however comprehensive knowledge of this multifaceted relationship remains elusive. Skeletal muscle is heterogeneous, comprised of slow-twitch (Type I) and fast-twitch (Type II) fibres, distinct in their metabolic and contractile properties. This study investigated the effects of varying glycogen availability whilst performing different modes of exercise in male endurance trained cyclists as previously described (Frankish et al., 2013). Single fibre western blotting was used to measure changes in protein abundance of specific glycogen related proteins.
Methods: The study was approved by the Human Ethics Committee at RMIT University. Seven male endurance trained cyclists performed two experimental trials in a randomized cross-over design (Table A). Following injection of 1% lidocaine (Xylocaine) into the skin/fascia, muscle biopsies were taken from the vastus lateralis at four time points (Figure) using the Bergstrom biopsy technique. Individual fibres were collected from freshly obtained tissue under paraffin oil, and prepared for western blotting analysis.
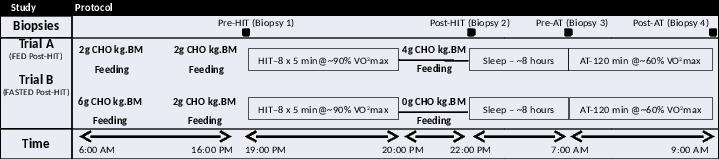
Figure. Study protocol outlining biopsy time points, carbohydrate supplementation and the high intensity (HIT) and aerobic (AT) exercise bouts.
Table. Fibre type specific abundance of glycogen proteins for Trial A and Trial B. Kruskal-Wallis test, Dunn’s multiple comparison test where *P<0.05 compared to Type I same bout of exercise, †P<0.05 compared to pre-exercise of same bout and same fibre type, ‡P<0.05 different from Pre-HIT of same fibre type. Values are mean ± SD normalized to Type I Pre-HIT within each trial.
| Protein abundance - Trial A (FED Post-HIT) | ||||||||
| Pre HIT | Post HIT | Pre AT | Post AT | |||||
| Protein | Type I | Type II | Type I | Type II | Type I | Type II | Type I | Type II |
| GS | 1 ± 0.3 | 0.9 ± 0.2 | 0.9 ± 0.3 | 0.8 ± 0.4 | 1.2 ± 0.3 | 1.2 ± 0.2 | 0.8 ± 0.3† | 0.8 ± 0.3† |
| phos-GS | 1 ± 0.5 | 1.2 ± 0.5 | 1.2 ± 0.7 | 1.1 ± 0.2 | 1 ± 0.2 | 0.3 ± 0.1*‡ | 0.6 ± 0.02 | 1.1 ± 1.5 |
| GBE | 1 ± 0.2 | 0.7 ± 0.2 | 1.2 ± 0.4 | 1 ± 0.3 | 1.1 ± 0.3 | 0.6 ± 0.2* | 0.6 ± 0.2† | 0.9 ± 0.3 |
| GP | 1 ± 0.2 | 2.8 ± 0.6* | 1 ± 0.2 | 1.7 ± 0.5*† | 1.1 ± 0.3 | 1.9 ± 0.7* | 0.5 ± 0.2† | 2 ± 0.4* |
| phos-GP | 1 ± 0.2 | 1.5 ± 0.9 | 1 ± 0.3 | 1.3 ± 0.4 | 0.9 ± 0.2 | 1.2 ± 0.9 | 0.4 ± 0.4 | 1 ± 0.5 |
| GDE | 1 ± 0.2 | 1.8 ± 0.7* | 1.1 ± 0.4 | 1.7 ± 0.5* | 1 ± 0.3 | 1 ± 0.3 | 0.7 ± 0.5 | 1.4 ± 0.3* |
| Protein abundance - Trial B (FASTED Post-HIT) | ||||||||
| Pre HIT | Post HIT | Pre AT | Post AT | |||||
| Protein | Type I | Type II | Type I | Type II | Type I | Type II | Type I | Type II |
Results: Following the HIT, fibre type- and trial-specific decreases in glycogen synthase (GS) and phosphorylase (GP), with concomitant changes in their phosphorylated forms. No changes in glycogen branching (GBE) or debranching enzyme (GDE) were seen following HIT in either trial. Following the AT in Trial A, there were fibre-type specific decreases in GS, GBE and GP (Table).
Conclusion: Glycogen availability, as well as fibre type recruitment during exercise influences the abundance of various glycogen related proteins, and does so in a fibre specific manner. These findings highlight the complexity of glycogen metabolism, and that fibre type needs to be considered when understanding its regulation.
Frankish BP, Lane SC, Areta JL, Stapleton DI, Hawley JA, Murphy RM. (2013). Single muscle fibre analysis of proteins important for glycogen metabolism in skeletal muscle from trained cyclists following varying bouts of exercise. Proc Aust Physiol Soc 44, 85P.